Density Lab
advertisement

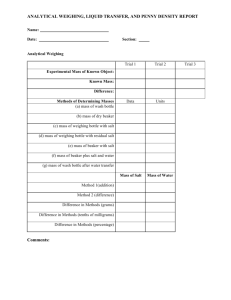
Experiment: Density Lab Preparation: Download “density lab.docx” and “density lab.xlsx” from http://www.mrjulien.com. Prepare a summary of the procedure and include it in the section marked Summary. Introduction to density Density (identified with the Greek letter ρ) is an intrinsic property of matter. This means that density depends only on the identity of the material. Density does not depend on the quantity of material or the form of the material. For example, the density of magnesium (Mg) metal is 1.738 g/cm3 at room temperature. Whether you have a handful of Mg pieces, a bucket full of Mg nanoparticles, or a dump truck full of Mg bricks the density is still 1.738 g/cm3. Density is the ratio of the mass of a material to its volume. The unit of density is usually given as g/cm3. Because 1 cm3 = 1 mL, it’s also common to see units of g/mL. Another common unit is kg/m3. To calculate the density of an unknown sample you need to know the mass of the sample and the volume of the sample. Equation (1) below can be used to calculate density. In this equation ρ is density in g/mL, m is mass in grams, and V is volume in mL. ρ = m/V (1) Density is a very easy property to measure and is often used in the laboratory to help determine the identity of an unknown sample or to determine the purity of a sample. For example, if someone handed you a ring and claimed that it was pure gold a simple measurement of the density of the ring would allow you to verify the claim. If your measured density were 19.30 g/cm3, then you would know the ring was pure gold. If the density were higher or lower than this value then you would know the ring was not pure. The density of water is 1.0 g/cm3. The density of gases is much lower than this value while the density of metals is greater than this value. When mixing compounds together, lower density materials rise above higher density materials. You observe this phenomenon every time you boil water or throw a stone into a lake. The density of the gas bubbles is less than the density of water, which causes them to rise to the top of the pot. The density of the stone is greater than water, which causes it to sink. Goals: When you leave the lab you should be able to: o Use an analytical balance to measure mass o Use a graduated cylinder and a burette to measure volume o Define density and know some of the ways it is applied in the lab o Calculate density Overview of the lab: In Part I, you will determine the density of a penny to see if it is made of pure copper. In Part II, you will determine the density of two different unknown solutions. You will learn how to measure mass using an analytical balance and how to measure volume using a graduated cylinder and a burette. Finally, you will conduct some basic error analysis to see if your density values are consistent with the known density of copper and the two unknown solutions. Part I: Density of pennies According to the US Treasury pennies are made from two metals in a proportion of 97.5% to 2.5%. New pennies minted in the U.S. have a diameter of 19.05 mm, a thickness of 1.55 mm, and weigh 2.500 grams. The color of a new penny suggests it is made of copper (Cu) metal but is Cu the dominant metal? To answer this question you will determine the density of a penny and compare the measured value to the known densities of a variety of metals. Equation (1) tells us that to calculate the density of a penny you must know its mass and volume. You will use an analytical balance to find the mass of the pennies. You will use two different methods to find the volume of the pennies. First, you will calculate the volume using the geometry of a new penny. Second, you will place the pennies into a graduated cylinder with a known volume of water and measure the increase in volume when you add pennies. This method is known as water displacement and is extremely accurate when done properly. Part II: Density of an unknown solution You will be given two unknown solutions. You will determine the density of each solution. To do this you will use a burette to deliver a known volume of the solution into a beaker. You will then use a balance to measure the mass of the solution you delivered. Procedures: Be sure to record units for all values and to record to as many decimals as possible in your Lab Report. Part I: 1) Use the diameter, thickness, and mass data provided by the U.S. Treasury to calculate the density of a new penny using equation (1). Record this calculation and your answer in your Lab Report (you may do this before lab to save time). 2) Count out 30 pennies. These need to be dry. 3) Weigh the pennies according to the following directions. Keep the pennies in the Same order as you weigh them. (Each penny should be approximately 2-3 grams.) a. Divide the pennies into three groups of five and one group of fifteen. b. Weigh each group of five and record the mass. c. For the group of fifteen, weigh each penny individually. In your Lab Report, you will have a data table that looks something like this: Penny Group 1 Pennies 1-5 Group 2 Pennies 6-10 Group 3 Pennies 11-15 Penny #16 Penny #17 Penny #18 Etc. until Penny #30 Mass (grams) 4) Record the mass of a single penny 10 times. This data will be used to determine the precision of your balance. Take a single penny and weigh it. Remove the penny from the balance, tare the balance, and then weigh the penny again. Repeat until you have weighed the same penny 10 times. 5) Fill your graduated cylinder to a convenient marking with water. The water should come to a fixed marking on the cylinder (e.g. exactly 36.0 or 42.0 ml). Remember to read the meniscus properly! Record the starting volume in your Lab Report. 6) Carefully add 15 pennies to the graduated cylinder (the three groups of five pennies). Try to avoid splashing water onto the sides of the cylinder, as this will bias your results. 7) Add pennies one by one (in the order you weighed them) until you reach an exact endpoint on a marked line (e.g. 57.0 or 63.0 mL). This can be any number of pennies greater than 15. AS AN EXAMPLE, your Lab Report sheet might look something like: 8) Final cylinder Initial cylinder reading (mL) reading (mL) 30.0 44.0 Number of pennies Mass of pennies (g) 22 67.764 Calculate the volume of the pennies in the graduated cylinder using the difference in the final and initial volumes. 9) You now know the total volume and total mass of the pennies in the graduated cylinder. Using this data calculate the density of pennies using equation (1). Remember: density is an intrinsic property. What matters is the ratio of the mass to the volume. The number of pennies you used in the cylinder does not matter when calculating the density. Part II: You will determine the density of two unknown solutions. You will use the same procedure for both solutions. You will measure volume using a burette and mass using a balance. 1) Pour ~100 mL of one unknown solution into a clean, dry beaker. 2) Rinse the burette with water: a. Make sure the stopcock is closed. b. Pour 5-10 mL of water into the burette. c. Place your finger over the open end of the burette and turn the burette upside down so that the water rinses the walls of the burette. Turn the burette back upright. d. Drain the water into the sink through the stopcock to rinse it out. 3) Rinse the burette with the unknown solution using the rinse procedure above. 4) Fill the burette with the unknown solution. DO NOT spend time trying to get the solution exactly to the top. (Remember- on a burette it’s the difference between where you start and end that is important). Record the initial volume. 5) Find a clean, dry 100 mL beaker. Weigh the empty beaker on the balance and record the mass. 6) Place the empty beaker underneath the burette. Dispense ~30 mL of the unknown solution into the beaker. Record the final burette reading in your Lab Report. 7) Reweigh the beaker and record the new mass in your Lab Report. 8) Repeat steps 3-6 two more times for your unknown (for a total of three trials). You do NOT need to empty and dry the beaker between each weighing. As long as you know the total volume and total mass of solution in the beaker you can calculate the density. 9) Repeat steps 3-7 for the second unknown solution. Make sure you rinse the burette and stopcock thoroughly with the new solution or you will get biased results. For each unknown solution you should have a data table in your Lab Report like the following: Trial number Initial beaker Initial burette Final burette Mass mass (g) reading (mL) reading (mL) beaker + solution (g) Make sure the data tables are clearly marked as to which unknown is being tested. Clean up and waste disposal: For Part I: The water in the graduated cylinder can be dumped down the drain. Dry any wet pennies with a paper towel. Place the paper towel in the trash and the pennies in the “wet penny” beaker at the front of the room. For Part II: Any leftover solution can be dumped in the Solution A Waste Container or the Solution B Waste Container, respectively. Rinse and dry any glassware you used and return the glassware to the way you found it at the start of the lab. Dry any spilled liquid on the lab bench with the paper towels provided. Before leaving: Clean up your lab area. The Lab Report will be due one week from the date that you collected the data. Be sure to answer all of the questions. Some questions have multiple parts. You must show your work for the calculations to receive credit. Lab report: Density Hand in this sheet with your answers. All values must include units and have the correct number of significant figures. Names __________________________________ Period _____ Date _____________________ Purpose: Procedure (Summary): Materials and Equipment: Calculations: (To receive credit you must show all of your work. Don’t forget to include both calculations for the volume of the penny and the calculations of density for the two solutions.) Data (To receive credit you must show charts in table form. You can cut and paste charts from Microsoft Excel into Microsoft Word. Don’t forget to include a chart for the mass of the three groups of pennies and the single pennies numbered 16-30, a chart for the same penny weighed 10 times. and a chart that shows initial and final cylinder readings with the mass of pennies included.) Part I: For the 15 pennies that you weighed individually: What is the average mass and one standard deviation (SD)? ______________________ What is the 95% Confidence Interval? = ______________________ (CI95% is the range calculated by ts/√N) Note that t = 2, N=15, s = SD According to the US Treasury a new penny weighs 2.500 grams. Is this value within the 95% CI that you calculated above? ___________ Is there any evidence that your pennies were worn? Explain. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ For the single penny you weighed repeatedly, what was the mean and standard deviation? (Use x±SD notation.) ________________ What was the total volume occupied by the pennies in the graduated cylinder? ______________ What was the total mass of these pennies?_________________ What is the density of the pennies? ______________________ What was the density you calculated using the U.S. Treasury data? ______________________ Look up density values for the following four metals and record them below. Report the source you used to find the values. Be sure to use a reputable source! (For example, the Merck Index or CRC Handbook). There are reputable online sources but random websites found with Google are not reputable sources! Be sure to include units. Cu ___________ Ni __________ Zn _______________ Ag _________________ Source _______________________________________________________________ What metal most closely corresponds to the density of your pennies? ________________ Data Part II: (To receive credit you must show charts in table form. You can cut and paste charts from Microsoft Excel into Microsoft Word. Don’t forget to include a chart for the average density and standard deviation of the two unknown solutions. Be sure to include units and correct number of sig figs!) Short answer questions: Answers to these questions should be typed. You may work with others to discuss the answers but all written work should be your own. 1) How does the density you measured using the water displacement method differ from the density you calculated based on the U.S. Treasury data? If they were the same, explain why. If they were different, what factors do you think contributed to the difference? 2) In Part I of the experiment we used water displacement to find the volume of the pennies. Would this method work for items that had a density less than 1.0 g/mL? Explain your answer. 3) You now know how to use a graduated cylinder and a burette. List some advantages and disadvantages of each. Why is a burette more precise than a graduated cylinder? 4) Lakes, ponds, and puddles freeze from the top down, which is good news for fish and ice skaters. Explain why this happens. Adapted from Jaekels, Experiment #2: Density, University of Washington.