Design a Lab
advertisement

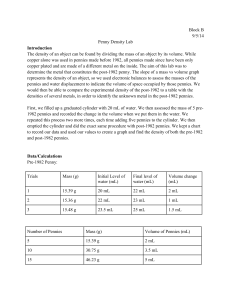
Design a Lab The Composition of Pennies BHS Lab Format You will use the BHS lab format for most of your labs in this class. Where is this found? You will also use the rubric to create an excellent final report! - You have used this for 2 years now…. Background U.S. pennies have been composed of copper and zinc since 1959 but the ratio of copper to zinc has changed due to an increase of copper prices. Copper and zinc are both metallic elements and they share many physical properties but have different densities. Density of copper is 9.0 g/mL Density of zinc is 7.1 g/mL Background (cont) Because of these density differences it is possible to track the changes in the composition of the penny. Your Task: Your job is to create and execute a lab procedure to figure out when the composition of the penny changed. 1st design the procedure using the BHS lab format and densities. This must be completed before the lab can be performed tomorrow. DUE today. 2nd execute the procedure and gather data Wednesday. DUE Wednesday 3rd The entire lab which includes a PEA conclusion (group effort)and analysis questions will be DUE on Friday. Further information This lab must use a non destructive method. You can’t destroy the pennies. You can (but may not need to) use the following equipment: Balance Beakers Graduated cylinder Funnel Forceps Test tubes Water You have 20 minutes to come up with a procedure! Further Information (cont) I will give you 10 pennies. Use the density equation to help you. Use the BHS lab format to shape your procedure and final report Key point…How can I get the volume of an “odd” shaped item??? What equation is needed to calculate density? Title: Penny Composition Problem, CH 1 Objectives: 1)To measure mass and volume, and calculate the density of a variety of pennies. 2)To determine approximately when the composition of the pennies changed. Procedure: 1) Record the mint date of each penny (10) in the data table. 2) Using the balance measure the mass (g) of each penny (10) and record. 3) Measure 15.0 mL of tap water in a graduated cylinder. Record the volume to the nearest 0.1 mL. 4) Add each penny to the water in the cylinder carefully. Tap out any air bubbles. Procedure (cont) 5) Measure the new volume of the water to the nearest 0.1 mL 6) Calculate the difference between initial and final volume 7) Divide the difference by the total number of pennies (10). This is the AVERAGE volume of one penny 8) Calculate the density of each penny by dividing the individual mass by the average volume. 9) Record these calculations in the data table. Questions: 1) Approximate the year that the United States Mint changed the composition of the penny. 2) What is the theoretical density of copper? Zinc? Use the CRC as your resource. Questions (cont) 3) Why might your density data be slightly different from the theoretical data? Why might your data be different from another group? How could we improve our results? 4) How would you determine the density of a similarly shaped solid object that was soluble in water? 5)If the density of iron is 7.86 g/mL, how many grams are there in 5.0 mL of iron metal? Use dimensional analysis to determine your answer.