ORGANIC CHEMISTRY - St.Joseph's College
advertisement

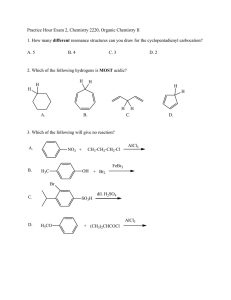
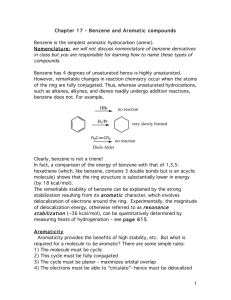
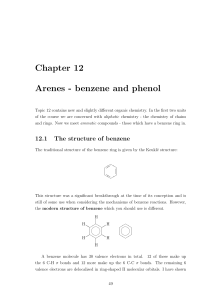
CLASS: M.Sc. CHEMISTRY 12N / 345 St. JOSEPH’S COLLEGE (AUTONOMOUS) TIRUCHIRAPPALLI – 620 002 SEMESTER EXAMINATIONS – NOVEMBER 2012 TIME: 3 Hrs. MAXIMUM MARKS: 100 SEM SET PAPER CODE TITLE OF THE PAPER I 2012 12PCH1101 ORGANIC CHEMISTRY – I SECTION – A Answer all the questions: 20 x 1 = 20 Choose the correct answer: 1. 2. 3. Which one is the correct structure of methane a) Tetrahedron c) Linear Which one is the correct Huckel’s rule a) (4n + 1) electrons c) (4n + 3) electrons Which carbocation is more stable a) primary c) tertiary b) d) Planar Octahedron b) d) (4n + 2) electrons (4n + 4) electrons b) d) secondary none of these 4. Which oxygen isotope is used for the labeling experiments a) O17 b) O18 c) O16 d) O19 5. Choose the correct acid for nitration of Benzene. a) HNO3 + H2SO4 b) c) HNO3 d) H2SO4 HNO3 + HCl Fill in the blanks: 6. NO2 is an example of ________ I effect. 7. Cycloheptatrienyl cation has ________ character. 8. Free radical is the ________ reactive reaction intermediate. 9. Radioactive disintegration is an example of ________ order reaction. 10. AlCl3 is used as a catalyst for ________ reaction. Answer in one or two sentences: 11. Define Dipole moment. 12. What is meant by chirality? 13. Predict the aromaticity of the following compounds. (ii) (i) 14. Write the resonance stabilization energy of real benzene. 15. Write the structure of Triplet carbene. 16. Give one example for neighbouring group participation. 17. What is meant by cross over experiment? 18. What is meant by order of a reaction? 19. What is Vilsmeir – Haack reaction? 20. What is sommelet reaction? SECTION – B Answer all the questions: 21. a. 5 x 6 = 30 What is hydrogen bonding? Explain different types of hydrogen bonding. OR 22. b. Explain the different conformations of Butane. a. Explain the terms with examples Alternant and non-alternant hydrocarbons. OR 23. b. Explain the Anisotropic effect in benzene. a. What is carbene? Write the two methods of generation of carbenes and give its reactions. OR 24. b. What is Hunsdiecker reaction? Explain its mechanism. a. Explain the intermediate and transition states with example. OR 25. b. Write any two applications of Hammett equation and Taft equation. a. What is Gattermann – Koch reaction? Explain its mechanism. OR b. What is Benzyne mechanism? Explain with example. SECTION – C Answer any FIVE questions: 26. Explain the following 5 x 10 = 50 (a) Inductive effect (b) Resonance effect (2) (2) (c) Steric effect (2) (d) Hyper conjugative effect (2) (e) Tautomerism 27. (2) Discuss the Aromatic character of the following with example. (i) Azulenes (3) (ii) Tropones (iii)Annulenes 28. (i) (3) (4) What is nitrine? Write any two methods of generation of reactions. (5) nitrines and give its (ii) What is kolbs reaction? Explain its mechanism. (5) 29. Discuss the influence of solvents, ionic strength and salt effect on the kinetics of the reaction. 30. (a) Explain the mechanism of the following reaction. (i) Sulphonation (3) (ii) Reimen - Tiemann reaction (3) (b) Discuss the effect of substrate structure and leaving group on mechanism. (4) 31. (i) Write one example for E and Z configuration. (ii) What is Craig’s rule (2) (iii)Write Hoffmann reaction (2) (iv) Write the rate constant equation for first order reaction (2) (v) What is SNAr mechanism. (2) ************** Benzyne (2)