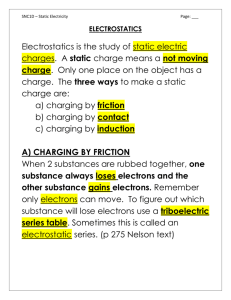
Electrostatic Series Chart
advertisement
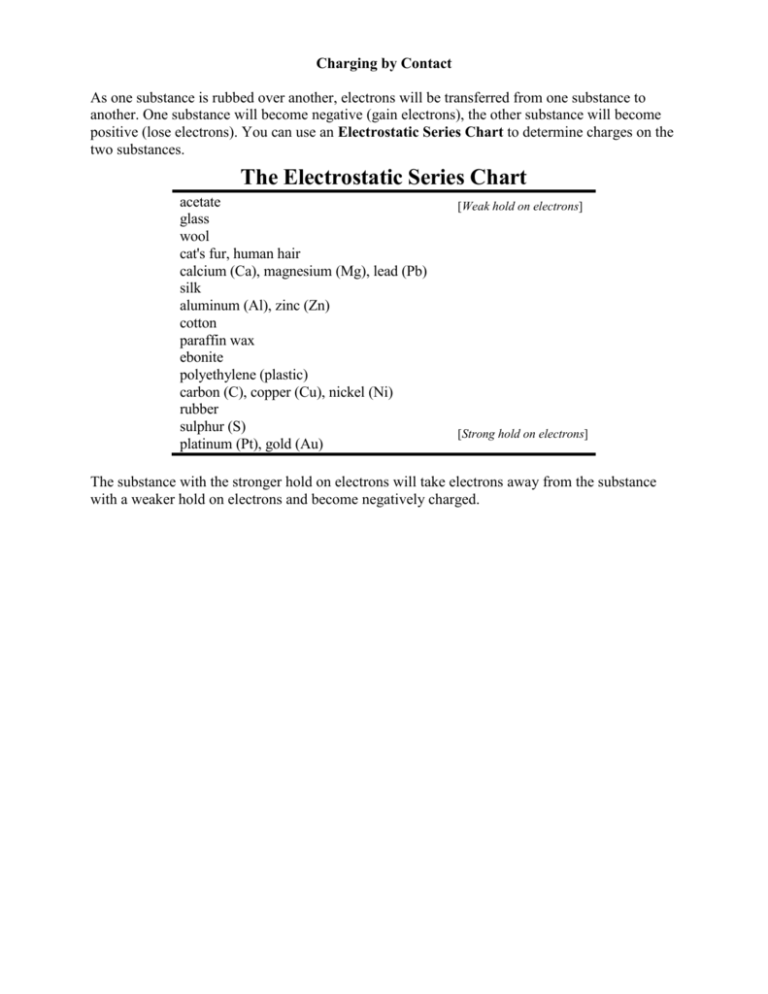
Charging by Contact As one substance is rubbed over another, electrons will be transferred from one substance to another. One substance will become negative (gain electrons), the other substance will become positive (lose electrons). You can use an Electrostatic Series Chart to determine charges on the two substances. The Electrostatic Series Chart acetate glass wool cat's fur, human hair calcium (Ca), magnesium (Mg), lead (Pb) silk aluminum (Al), zinc (Zn) cotton paraffin wax ebonite polyethylene (plastic) carbon (C), copper (Cu), nickel (Ni) rubber sulphur (S) platinum (Pt), gold (Au) [Weak hold on electrons] [Strong hold on electrons] The substance with the stronger hold on electrons will take electrons away from the substance with a weaker hold on electrons and become negatively charged.