SCH 4u Naming Aromatic Compounds
advertisement

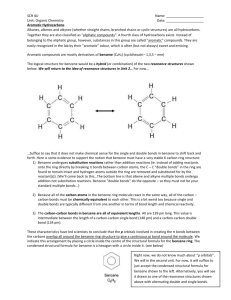
Aromatic Compounds Benzene is the simplest aromatic compound Molecular formula is C6H6 o Due to the formula scientists thought that it had 2 double bonds and a triple bond o But, it doesn’t react like other compounds with double and triple bonds. Has a ring structure with 3 single bonds and 3 double bonds (6 carbon atoms) 2 methods of drawing a benzene ring. What makes benzene special? o The electrons that form the double bonds are shared by the entire ring. o Therefore, benzene has 6 identical bonds that are halfway b/n single and double bonds. o These bonds are more stable than regular double bonds and give benzene special properties. Aromatic Compounds – all have this special form of electron sharing. Naming Aromatic Hydrocarbons 1. Number the carbons in the benzene ring, starting with a carbon attached to a functional group. If there is more than one functional group, start with the most important/complex one. 2. Name any branches. Give the branches position numbers. If there is only one branch, you do not need to number it (by convention it will be 1). 3. Just like the aliphatic hydrocarbons, write the position number and name for each branch as a prefix before the root. 4. The root for all of these is benzene. Functional groups ranked by importance or priority: Highest Lowest -OH -NH2 -F, -Cl, -Br, -I -propyl -ethyl -methyl Ortho, Meta and Para prefixes: Often used instead of branch numbers when there are only 2 branches. o Ortho – the branches are next to each other on the ring. o Meta – the branches are one carbon apart on the ring. o Para – the branches are opposite each other on the ring. 1,2-dimethylbenzene = ortho-dimethylbenzene (ortho-xylene) 1,3-dimethylbenzene = meta-dimethylbenzene (meta-xylene) 1,4-dimethylbenzene = para-dimethylbenzene (para-xylene)