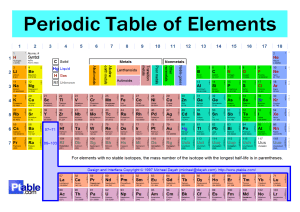
Unit 2 From atoms to molecules Periodic Table: Electronic configuration Chemistry MYP4 2021/22 n° neutrons + n° protons (+) = Mass Number Atomic Number = n° protons (+) = n° electrons (-) (is the number on the periodic table) The Atom is made of subatomic particles: - Protons: in the nucleus, positive charge - Neutrons: in the nucleus, no charge - Electrons: moving around the nucleus, negative charge Mass 1,672 × 10-24 g 0,000000000000000000000001672 g 1,672 × 10-24 g 0,000000000000000000000001672 g 9,109 × 10-27 g 0,000000000000000000000000009109 g Since the mass value of the electrons (-) is negligible versus the mass value of the other particles, the mass in the atom is given only by the particles in the nucleus: protons (+) and neutrons. Rutheford model (planetary model) The electrons are moving in orbits around the nucleus and the orbits are at a fixed distance from the nucleus (defined also as "shells") In the very 1st shell, there could be only 2 electrons. And there are 8 electrons for any other shell starting from the 2nd one. Electrons in the most external orbit (outer shell) are very important to deter mine chemical and physical properties. Electrons in the outer shell are called VALENCE ELECTRONS. The number of electrons/protons is increasing one by one along the periodic table from left to right and row by row. Elements in the same group have the same valence electron number (represented by the number of the group) Periodic table The periodic table arranges all the elements in groups according to their properties. The horizontal rows are called periods and are labeled from 1 to 7. The vertical columns are called groups are labeled from 1 to 8 (or 18). G r o u p It is like playing Battleship! period Hydrogen 1 electron (-) 1 proton (+) in the nucleus Only one shell 1st shell is the outer shell One electron in the outer shell Period 1 Group 1 He - Helium 2 electrons (-) 2 protons (+) 2 neutrons in the nucleus Only one shell Two electron in the outer shell 1st shell is the outer shell and it is full Period 1 Group 8 Exception! It should be group 2 but... It goes in Group 8 because it is the group with full outer shells 3 electrons (-) 3 proton (+) 4 neutrons Li - Lithium in the nucleus Z = Mass Number = 7 Two total shells. One electron in the outer shell. Three total electrons. Period 2 Group 1 A = Atomic number = 3 4 electrons (-) Be - Berillium 4 protons (+) in the nucleus 5 Neutrons Z = Mass Number = 9 Two total shells. Two electron in the outer shell. Four total electrons. Period 2 Group 2 A = Atomic number = 4 5 electrons (-) B - Boron __ Protons (+) in the nucleus __ Neutrons ? Z = Mass Number = Two total shells. Three electron in the outer shell. Five total electrons. Period 2 Group 3 A = Atomic number = ? 5 electrons (-) B - Boron 5 Protons (+) in the nucleus 6 Neutrons Z = Mass Number = 11 Two total shells. Three electron in the outer shell. Five total electrons. Period 2 Group 3 A = Atomic number = 5 6 electrons (-) C- Carbon __ Protons (+) in the nucleus __ Neutrons ? Z = Mass Number = Two total shells. Four electron in the outer shell. Six total electrons. Period 2 Group 4 A = Atomic number = ? HOMEWORK Design at the same way the electronic configuration of the atoms from Nitrogen to Magnesium Symbols Na Sodium (from Natrium, lat.) K Potassium (from Kalium, lat.) Cu Copper (from Cuprum, lat. "from Cyprus", where the major part of this metal was extracted at the time) Au Gold (from Aurum, lat.) Ag Silver (from Argentum, lat.) Pb Lead (from Plumbum, lat.) Hg Mercury (from Hydrargirium, gr. "silver water") W Tungsten (from Wolframio/Wolf ram, ted. "wolf black" or Tung sten, sved. "heavy stone") Sb Antimony (from Stibium, lat. "stick") Formula one or more symbols with numerical subscripts that represents the single unit atomic composition of the fundamental particle that makes up the substance. Elements: Monatomic: The formula is the same as the symbol, eg. He, Ar, Na. Diatomic: When the natural form in which the element exists is made by two atoms of the same element: H2, O2, N2, Cl2, F2, I2, Br2 Compounds: Formula is given by the symbols of each atom in the molecule together with numerical subscripts that mean the quantity of each atom forming the molecule. e.g. CO2 = one atom of Carbon and two atoms of Oxygen Classification Elements are classified in three big families: Metal Metalloid/Semi metal Non-metal The physical and chemical properties depend on the valence of the elements. The valence of the elements is the number electrons that the atom has on the outer shell and is directly connected to the number of bonds that the atom is able to form with a standard element and it is closely correlated with position in the periodic table. Elements are divided in groups by their valence electrons number Groups Alkali Metals Alkaline Earth Metals 1st column on the periodic table (Group 1) not including hydrogen. Second column on the periodic table. (Group 2) Very reactive metals, always combined with something else in nature (like in salt). Reactive metals that are always combined with nonmetals in nature. They form cations (positive ions) Soft metals with low melting points They BOTH form compounds with alkaline properties (Hydroxides). Most reactive elements together with Group 7 Halogens. Several of these elements are important mineral nutrients (such as Na, K, Mg and Ca) very important for the maintenance and regulation of the body physiology. Halogens The Noble Gases Elements in group 8 have a full valence shell. monatomic gases Elements in group 7. Very reactive, volatile, diatomic, nonmetals Always found combined with other element in nature. They form anions (negative ions) Transition Metals VERY stable, inert = unreactive (very suitable in applications where reactions are not wanted) (i.e. Used in lighted “neon” signs) • good conductors of heat and electricity. • they can be hammered or bent into shape easily (ductility). • high melting points (but mercury is a liquid at room temperature) • usually hard and tough. • high densities. Non Metals and semi-metals Group 3 Boron is commonly classified as a (metalloid) while the rest are considered post-transition metals. Carbon family. Carbon is the building block of organic chemistry together with Hydrogen. Silicon and Germanium are important semiconductors. Group 5 Group 4 Nitrogen family. Nitrogen is the main component of the air together with Oxygen (78% and 20%) and Argon, CO2, etc… The tip of matches is made of phosphorus. Oxygen family. Elements of this group are called "chalcogens" that means "ore formers" from the greek. Selenium has some biological functions (in aminoacids) but it is also toxic. Tellurium and Polonium are toxic too; Polonium has also a radioactive isotope. Group 6