The Periodic Table Elements RULE!
advertisement

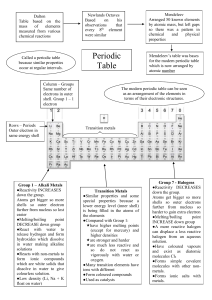
The Periodic Table Elements RULE! Remember... • Matter: Anything that has a mass and takes up space Matter is composed of: • Elements!!!!! Element • Material that cannot be broken down into new material that is stable or more simple through chemical reactions. • Has a specific set of properties • Hydrogen, Oxygen, Gold, Neon... • 117 elements exist • 94 of them occur naturally Atom • The smallest amount of an element (or substance) that still has all of the properties of the element. • Cut a piece of aluminum...how many cuts can you make and still have Al? Gold...As an Example 3057473598700243704305 atoms!!!! What does an element look like? Made of Protons, Electrons and Neutrons That is DRAWN MENDELEEV! 1869- He Came up with a way to organize the elements The Periodic Table A table organized by similarities and differences among elements Periods Elements in the same period have the same number of atomic orbitals (electron cloud) How many shells should Li have? Guess... Good! Groups • The Columns in the Periodic Table • Number of electrons in outer shell • Transition metals are drawn • Group 1 has one electron in outer shell • How many in Boron? Boron is NOT boring Color your table as follows: Solid Liquid Gas Metals/Non-Metal/Metalloid Table link