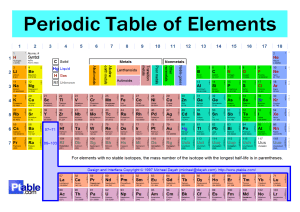
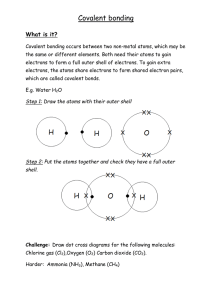
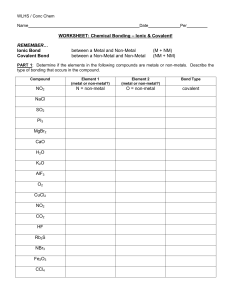
Name of Ionic Compound Draw the outer shell of the metal (use dots) Write the number of electrons to be lost Write the charge on the metal ion Draw the outer shell of the non-metal (use crosses) Write the number of electrons to be gained Write the charge on the non-metal ion Draw the new outer shells now they have lost/gained electrons Sodium Chloride Magnesium Oxide Beryllium Oxide Success Criteria – Does your final answer contain the following? (Tick off) Square brackets around the ions Charges of both ions shown in the right hand corner (outside of the bracket) Any gained electrons are shown as dots (not crosses) on your non-metal