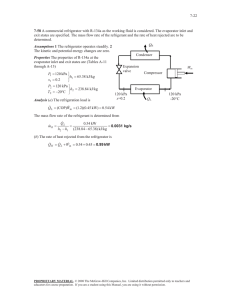
8-27 determined. are no work interactions involved other than the boundary work.
advertisement
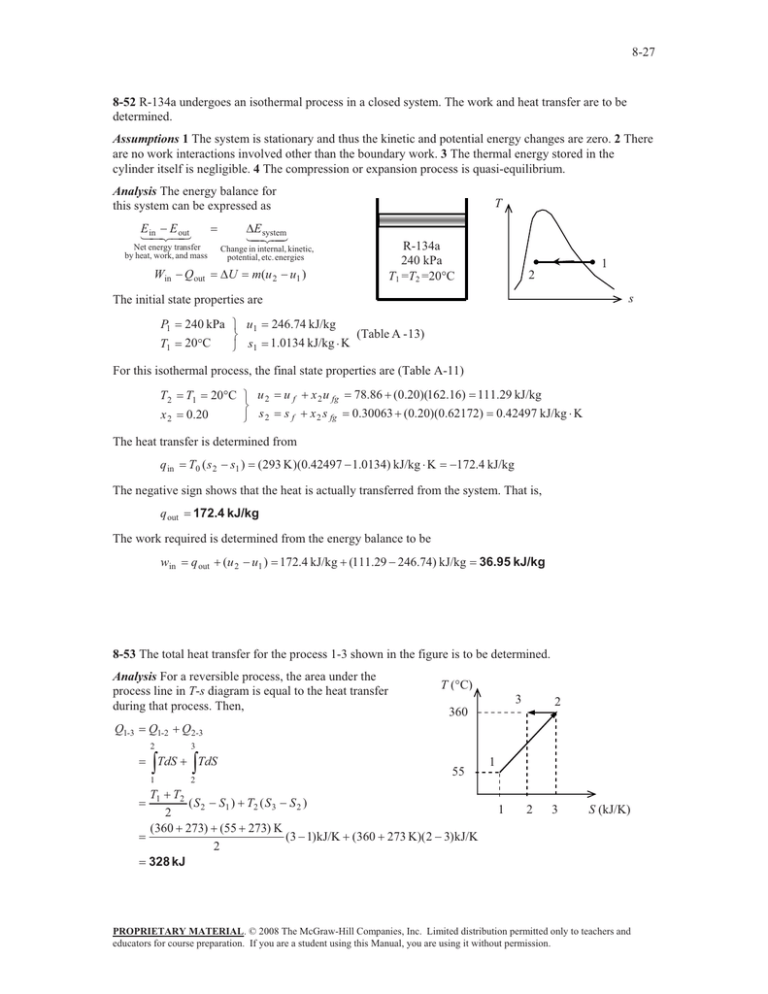
8-27 8-52 R-134a undergoes an isothermal process in a closed system. The work and heat transfer are to be determined. Assumptions 1 The system is stationary and thus the kinetic and potential energy changes are zero. 2 There are no work interactions involved other than the boundary work. 3 The thermal energy stored in the cylinder itself is negligible. 4 The compression or expansion process is quasi-equilibrium. Analysis The energy balance for this system can be expressed as E E in out Net energy transfer by heat, work, and mass Win Qout T 'E system R-134a 240 kPa T1 =T2 =20qC Change in internal, kinetic, potential, etc. energies 'U m(u 2 u1 ) 1 2 s The initial state properties are P1 T1 240 kPa ½ u1 ¾ 20qC ¿ s1 246.74 kJ/kg 1.0134 kJ/kg K (Table A - 13) For this isothermal process, the final state properties are (Table A-11) T2 x2 20qC ½ u 2 ¾ 0.20 ¿ s2 T1 u f x 2 u fg 78.86 (0.20)(162.16) 111.29 kJ/kg s f x 2 s fg 0.30063 (0.20)(0.62172) 0.42497 kJ/kg K The heat transfer is determined from q in T0 ( s 2 s1 ) (293 K )(0.42497 1.0134) kJ/kg K 172.4 kJ/kg The negative sign shows that the heat is actually transferred from the system. That is, q out 172.4 kJ/kg The work required is determined from the energy balance to be win q out (u 2 u1 ) 172.4 kJ/kg (111.29 246.74) kJ/kg 36.95 kJ/kg 8-53 The total heat transfer for the process 1-3 shown in the figure is to be determined. Analysis For a reversible process, the area under the process line in T-s diagram is equal to the heat transfer during that process. Then, Q1-3 T (qC) 3 2 360 Q1-2 Q2-3 2 ³ 1 3 ³ TdS TdS 2 55 T1 T2 ( S 2 S1 ) T2 ( S 3 S 2 ) 2 (360 273) (55 273) K (3 1) kJ/K (360 273 K)( 2 3) kJ/K 2 328 kJ 1 1 2 3 S (kJ/K) PROPRIETARY MATERIAL. © 2008 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.