Classification using micro
advertisement

Whole Genome Assembly
Mate Pairs
• Mate-pairs allow you to merge islands
(contigs) into super-contigs
Super-contigs are quite large
• Make clones of truly predictable length. EX: 3
sets can be used: 2Kb, 10Kb and 50Kb. The
variance in these lengths should be small.
• Use the mate-pairs to order and orient the
contigs, and make super-contigs.
Problem 3: Repeats
Repeats & Chimerisms
• 40-50% of the human
genome is made up of
repetitive elements.
• Repeats can cause great
problems in the
assembly!
• Chimerism causes a
clone to be from two
different parts of the
genome. Can again give a
completely wrong
assembly
Repeat detection
• Lander Waterman strikes again!
• The expected number of clones in a Repeat containing island
is MUCH larger than in a non-repeat containing island
(contig).
• Thus, every contig can be marked as Unique, or non-unique.
In the first step, throw away the non-unique islands.
Repeat
Detecting Repeat Contigs 1: Read Density
• Compute the log-odds
ratio of two
hypotheses:
• H1: The contig is from
a unique region of the
genome.
• The contig is from a
region that is
repeated at least
twice
Detecting Chimeric reads
•
•
•
•
Chimeric reads: Reads that
contain sequence from two
genomic locations.
Good overlaps: G(a,b) if a,b
overlap with a high score
Transitive overlap: T(a,c) if
G(a,b), and G(b,c)
Find a point x across which only
transitive overlaps occur. X is a
point of chimerism
Contig assembly
•
•
Reads are merged into contigs
upto repeat boundaries.
(a,b) & (a,c) overlap, (b,c) should
overlap as well. Also,
–
•
•
shift(a,c)=shift(a,b)+shift(b,c)
Most of the contigs are unique
pieces of the genome, and end at
some Repeat boundary.
Some contigs might be entirely
within repeats. These must be
detected
Creating Super Contigs
Supercontig assembly
• Supercontigs are built incrementally
• Initially, each contig is a supercontig.
• In each round, a pair of super-contigs is merged
until no more can be performed.
• Create a Priority Queue with a score for every
pair of ‘mergeable supercontigs’.
– Score has two terms:
• A reward for multiple mate-pair links
• A penalty for distance between the links.
Supercontig merging
• Remove the top scoring pair (S1,S2) from
the priority queue.
• Merge (S1,S2) to form contig T.
• Remove all pairs in Q containing S1 or S2
• Find all supercontigs W that share matepair links with T and insert (T,W) into the
priority queue.
• Detect Repeated Supercontigs and remove
Repeat Supercontigs
• If the distance between
two super-contigs is not
correct, they are marked
as Repeated
• If transitivity is not
maintained, then there is
a Repeat
Filling gaps in Supercontigs
Consenus Derivation & Assembly
• Summary
– Do an “all pairs” prefix-suffix alignment. (Speedup using
k-mer hashing).
– Construct a graph of overlapping alignments.
– Break the graph into “unique” regions (Number of clones
similar to prediction using LW), and “repeat/chimeric”
regions. Each such “unique’ region is called a contig.
– Merge contigs into super-contigs using mate-pair links
– For each contig, construct a multiple alignment, and
consensus sequence.
– Pad the consensus sequence using NNs.
Summary
• Once controversial, whole genome shotgun is now routine:
–
–
–
–
Human, Mouse, Rat, Dog, Chimpanzee..
Many Prokaryotes (One can be sequenced in a day)
Plant genomes: Arabidopsis, Rice
Model organisms: Worm, Fly, Yeast
• WGS must be followed up with a finishing effort.
• A lot is not known about genome structure, organization and
function.
– Comparative genomics offers low hanging fruit.
Biol. Data analysis: Review
Assembly
Sequence
Analysis/
DNA signals
Gene Finding
Protein
Sequence
Analysis
Other static analysis is possible
Genomic
Analysis/
Pop.
Genetics
Assembly
Protein
Sequence
Analysis
Sequence
Analysis
Gene Finding
ncRNA
A Static picture of the cell is insufficient
•
Each Cell is continuously
active,
–
–
–
–
•
Genes are being
transcribed into RNA
RNA is translated into
proteins
Proteins are PT modified
and transported
Proteins perform various
cellular functions
Can we probe the Cell
dynamically?
–
–
–
Which transcripts are
active?
Which proteins are active?
Which proteins interact?
Gene
Regulation Transcript
profiling
Proteomic
profiling
Micro-array analysis
The Biological Problem
• Two conditions that need to be
differentiated, (Have different
treatments).
• EX: ALL (Acute Lymphocytic Leukemia) &
AML (Acute Myelogenous Leukima)
• Possibly, the set of genes over-expressed
are different in the two conditions
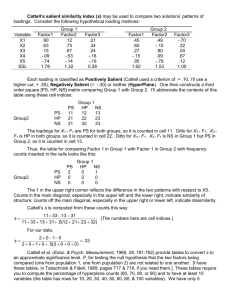
Supplementary fig. 2. Expression levels of predictive genes in independent dataset. The expression levels of the 50
genes most highly correlated with the ALL-AML distinction in the initial dataset were determined in the independent
dataset. Each row corresponds to a gene, with the columns corresponding to expression levels in different samples.
The expression level of each gene in the independent dataset is shown relative to the mean of expression levels for
that gene in the initial dataset. Expression levels greater than the mean are shaded in red, and those below the mean
are shaded in blue. The scale indicates standard deviations above or below the mean. The top panel shows genes
highly expressed in ALL, the bottom panel shows genes more highly expressed in AML.
Gene Expression Data
• Gene Expression data:
s1 s2
– Each row corresponds to a gene
– Each column corresponds to an
expression value
• Can we separate the experiments
into two or more classes?
• Given a training set of two
classes, can we build a classifier
that places a new experiment in
one of the two classes.
g
s
Three types of analysis problems
• Cluster analysis/unsupervised learning
• Classification into known classes
(Supervised)
• Identification of “marker” genes that
characterize different tumor classes
Supervised Classification: Basics
• Consider genes g1 and g2
– g1 is up-regulated in class A, and down-regulated in class B.
– g2 is up-regulated in class A, and down-regulated in class B.
• Intuitively, g1 and g2 are effective in classifying the two
samples. The samples are linearly separable.
1
2
3
4
5
6
g1 1 .9 .8 .1 .2 .1
g2 .1 0 .2 .8 .7 .9
1
2
3
Basics
• With 3 genes, a plane is used to separate (linearly
separable samples). In higher dimensions, a
hyperplane is used.
Non-linear separability
• Sometimes, the data is
not linearly separable,
but can be separated by
some other function
• In general, the linearly
separable problem is
computationally easier.
Formalizing of the classification
problem for micro-arrays
• Each experiment (sample) is
a vector of expression
values.
– By default, all vectors v are
column vectors.
– vT is the transpose of a vector
• The genes are the dimension
of a vector.
• Classification problem: Find
a surface that will separate
the classes
v
vT
Formalizing Classification
• Classification problem: Find a surface (hyperplane)
that will separate the classes
• Given a new sample point, its class is then
determined by which side of the surface it lies on.
• How do we find the hyperplane? How do we find
the side that a point lies on?
1 2 3
g1
g2
1
4 5 6
1 .9 .8 .1 .2 .1
.1 0 .2 .8 .7 .9
2
3
Basic geometry
• What is ||x||2 ?
• What is x/||x||
• Dot product?
x=(x1,x2)
xT y
x1 y1 x 2 y 2
|| x || || y || cos x cos y || x || || y || sin( x )sin( y )
|| x || || y || cos( x y )
y
Dot Product
x
• Let be the unit vector.
– |||| = 1
• Recall that
– Tx = ||x|| cos
Tx
• What is
if x is
orthogonal (perpendicular)
to ?
• How do we specify a
hyperplane?
Tx = ||x|| cos
Hyperplane
• How can we define a
hyperplane L?
• Find the unit vector that
is perpendicular (normal
to the hyperplane)
Points on the hyperplane
• Consider a hyperplane L
defined by unit vector ,
and distance 0
• Notes;
xT
– For all x L,
must be
the same, xT = 0
– For any two points x1, x2,
• (x1- x2)T =0
x2
x1
Hyperplane properties
• Given an arbitrary point x,
what is the distance from x
to the plane L?
– D(x,L) = (Tx - 0)
• When are points x1 and x2
on different sides of the
hyperplane?
x
0
Separating by a hyperplane
• Input: A training set of +ve &
-ve examples
• Goal: Find a hyperplane that
separates the two classes.
• Classification: A new point x
is +ve if it lies on the +ve side
of the hyperplane, -ve
otherwise.
• The hyperplane is
represented by the line
• {x:-0+1x1+2x2=0}
+
x2
x1
Error in classification
• An arbitrarily chosen
hyperplane might not separate
the test. We need to minimize
a mis-classification error
• Error: sum of distances of the
misclassified points.
• Let yi=1 for +ve example i, yi=-1
otherwise.
•
y x
Other definitions are also
D(, 0 )
possible.
i
iM
T
i
0
+
x2
x1
Gradient Descent
• The function D() defines
the error.
• We follow an iterative
refinement. In each step,
refine so the error is
reduced.
• Gradient descent is an
approach to such iterative
refinement.
D'()
D()
D’()
Rosenblatt’s perceptron learning
algorithm
D(, 0 )
y x
i
T
i
0
iM
D(, 0 )
yi xi
iM
D(, 0 )
yi
0
iM
y i x i
Update rule :
y
0 0 i
Classification based on perceptron
learning
• Use Rosenblatt’s algorithm to compute the
hyperplane L=(,0).
• Assign x to class 1 if f(x) >= 0, and to class
2 otherwise.
Perceptron learning
• If many solutions are possible, it does no
choose between solutions
• If data is not linearly separable, it does
not terminate, and it is hard to detect.
• Time of convergence is not well understood
Linear Discriminant analysis
•
•
•
Provides an alternative
approach to classification
with a linear function.
Project all points, including
the means, onto vector .
We want to choose such
that
– Difference of projected
means is large.
– Variance within group is
small
+
x2
x1
LDA Cont’d
m˜ 1
1
T x w T m1
n1 x
Scatter between samples : | m˜ 1 m˜ 2 | (m1 m2 )
2
T
m˜ 1 m˜ 2 T SB
2
scatter within sample : s˜12 s˜22
where, s˜12 (y m˜ 1 ) 2
y
(
T
(x m1 )) 2 T S1
x D1
s˜12 s˜22 T (S1 S2 ) T Sw
T
Fisher Criterion max T SB
Sw
2
Maximum Likelihood discrimination
• Suppose we knew the
distribution of points in
each class.
– We can compute Pr(x|i)
for all classes i, and take
the maximum
ML discrimination
• Suppose all the points
were in 1 dimension, and
all classes were normally
distributed.
Pr( i | x)
Pr(x | i )Pr( i )
Pr(x | j )Pr( j )
j
gi (x) ln Pr(x | i ) ln Pr( i )
(x i ) 2
ln Pr( i )
2
2 i
ML discrimination recipe
• We know the distribution for each class, but not
the parameters
• Estimate the mean and variance for each class.
• For a new point x, compute the discrimination
function gi(x) for each class i.
• Choose argmaxi gi(x) as the class for x