Density Lab- Chemistry in the Community
advertisement

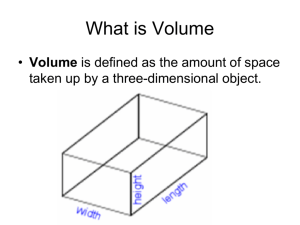
Name____________________________________________ Class________ Date___________ Lab Partner______________________________________ Density Lab- Chemistry in the Community Introduction: This lab highlights the following important points: Density is defined as the mass of an object per unit of volume: 𝑚𝑎𝑠𝑠 𝐷𝑒𝑛𝑠𝑖𝑡𝑦 = 𝑣𝑜𝑙𝑢𝑚𝑒 Density is an intensive property, meaning that for a given substance, it is constant, and does not depend on mass. Density can be used as a characteristic of a substance. Purpose: The purpose of this lab is to determine the density of pennies from "Set A" and "Set B." Notes: Do NOT mix up the pennies from Sets A and B. Procedure: Part A: Penny Set A 1. Record (in the Data Section) the US Mint years on each of the pennies in Set A. 2. Determine the mass of 5 pennies from Set A using an electronic balance. Record this mass in Data Table 1. 3. Add 5 more pennies to the first group and determine the mass of these 10 pennies. Record this mass. 4. Repeat step2, each time adding 5 more pennies to those already on the balance, until all 25 pennies have been used. 5. Fill a 100-mL graduated cylinder to the 20.0-mL mark with water. Read the bottom of the meniscus to measure the water level. 6. Still working with the same set of pennies, gently drop the FIRST group of 5 pennies into the graduated cylinder. Record the total water level in the appropriate data table. 7. Add the SECOND group of 5 pennies to the graduated cylinder, making a total of 10 pennies. Record the new total water level. 8. Repeat step 6 until all 25 pennies have been added to the graduated cylinder. 9. Discard the water in the graduated cylinder. Dry the pennies with a paper towel and put the dry pennies back into their container. Do not mix the Set A pennies with the Set B pennies. Part B: Penny Set B 1. Record (in the Data Section) the US Mint years on each of the pennies in Set B. 2. Repeat the procedure for Part II, recording your data in Data Table 2. Procedure for Making Graphs in Microsoft Excel To analyze the data, you will graph your data using Microsoft Excel. 1. Enter the data from Data Table 1 in a spreadsheet in Excel. Enter “Volume” of the pennies in column B and “Mass” of the pennies in column C. 2. Using the data from Step 1, create a scatter plot with “Mass” on the y-axis and “Volume” on the x-axis. Do this by shading the Data Table 1 data. Then, click “Charts” on the green toolbar above. Choose “Other,” and then “Marked Scatter.” A scatter plot should appear. Edit your graph so that it has a title, axis titles, and units on the axes of your graph. 3. Create a line of best fit. Do this by clicking on a data point on the graph, and then right clicking (on a Mac, this is CTRL + click). Choose “Add Trendline.” In the resulting dialog box, choose “Options.” Click “Display equation on chart.” This will display the equation for the line of best fit on the graph. 4. Record the equation for the line of best fit (there is a space to do this on your Data Sheet). In your line of best fit, the slope is your density for that set. (in the y = mx+b form, “m” is your slope). Name________________________________________Lab Partner_______________________ Density Lab Data Part A, Set A US Mint Years: Data Table 1 A B C D E Number of Pennies Mass of Pennies (g) Total Volume in the Cylinder (mL) Volume of the Pennies = Total Volume – 20mL Density of the Pennies 𝑚𝑎𝑠𝑠 (𝑐𝑜𝑙𝑢𝑚𝑛 𝐵) 𝐷𝑒𝑛𝑠𝑖𝑡𝑦 = 𝑣𝑜𝑙𝑢𝑚𝑒 (𝑐𝑜𝑙𝑢𝑚𝑛 𝐷) 5 10 15 20 25 g/mL g/mL g/mL g/mL g/mL Part B, Set B US Mint Years: Data Table 2 A Number of Pennies 5 10 15 20 25 B Mass of Pennies (g) C Total Volume in the Cylinder (mL) D Volume of the Pennies = Total Volume – 20mL E Density of the Pennies 𝑚𝑎𝑠𝑠 (𝑐𝑜𝑙𝑢𝑚𝑛 𝐵) 𝐷𝑒𝑛𝑠𝑖𝑡𝑦 = 𝑣𝑜𝑙𝑢𝑚𝑒 (𝑐𝑜𝑙𝑢𝑚𝑛 𝐷) g/mL g/mL g/mL g/mL g/mL Discussion and Graphs: 1. In the space below, draw the graphs that you produced in Microsoft Excel Graph A Graph B 2. According to your data, what was the density of the Set A pennies? 3. According to your data, what was the density of the Set B pennies? 4. How, if at all, do the US Mint years relate to the densities? 5. Explain, in terms of density, why pennies sink in water. 6. Density is an intrinsic property. This means that the density of an object is NOT related to the size of the object, or how many of the object there are. How does this relate to your results?