ASCLS GA 2015 Genetic Testing
advertisement

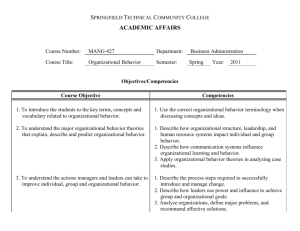
Laboratory Competencies: Promoting Sustainability John Ridderhof, DrPH, HCLD (ABB) Senior Advisor for Laboratory Integration Division of Laboratory System/CSELS ASCLS GA Annual Meeting May 16, 2015 Marietta Georgia Center for Surveillance, Epidemiology, and Laboratory Services Office of the Director CDC’s Role “Public health laboratories are critically important to the health of their communities and the entire nation. We must do all we can to ensure that the public health laboratory system maintains its capacity to address today’s health threats and those of the future.” – Dr. Frieden, A Practical Guide To Assessing and Planning Implementation of Public Health Laboratory Service Changes, May 2012 CDC’s Laboratory Activities Major Functions/Focal Areas • Outbreak assistance • Reference laboratory testing • Management and inventory of specimens • Materials, reagents, equipment • Research collaboration, technology development, transfer, and evaluation • Quality assurance, standards, guidelines, and proficiency testing CDC’s Laboratory Activities Major Functions/Focal Areas • Building infrastructure and laboratory capacity • Facilities design and construction • Safety and security • Training and workforce development • Laboratory information management systems • Policy and partnership development, advocacy • Strengthening public health testing capacity and integration with clinical labs CDC is the reference laboratory for the country and the world Infectious diseases (pathogen reference, diagnosis, research) Environmental health (genetics, nutrition, chemicals, toxins) Preparedness and response (bioterrorism, outbreaks, disasters) Occupational safety and health (workplace safety) Lab standards and science (quality & laboratory standardization) Global health (HIV, malaria, TB, emerging/chronic diseases) Improving Emergency Response More than 160 public health, military, federal, food, veterinary, and international laboratories Food safety in the United States • Food-borne outbreaks are common and costly – About 1,000 outbreaks/year – 48M people/year get sick from eating contaminated food and 3,000 die – Cost to society as high as $152B/year • CDC’s role in food safety – Identify and stop outbreaks – Track trends in food-borne illness and outbreaks – Conduct applied research for better diagnosis and prevention – Track effectiveness of policies to reduce infections Newborn Screening Quality Assurance Program Services provided Filter paper evaluation Reference materials Quality control materials Proficiency testing Training, consultations, network resources Partners Association of Public Health Laboratories (APHL) 73 domestic screening laboratories Laboratories in 54 countries 400 plus screening laboratories worldwide Emergency operations • • Coordinates CDC’s preparedness and emergency response activities for public health emergencies Emergency Operations Center monitors and coordinates response activities during public health emergencies CDC Ebola Response Clinical laboratories play a vital role in Laboratory networks 160+ public health, military, federal, food, veterinary, and internatioal labs Influenza-like illness monitoring network Tracking Multiple Drug resistant TB CDC measures 300+ environmental chemicals in blood and urine State Public Health Labs How CDC helps states…. Reference testing Methods development and transfer Reagents External quality assessment Technical consultation Domestic global networks (LRN, Pulsenet) Guidelines and recommendations Direct funding APHL Non-profit organization More than 80 Staff Growing membership ~800 members State and Local Public Health Labs • Leadership, delegates State Environmental Laboratories Agricultural Laboratories Individual Members Roles of Health Laboratories Provide information for decision making Clinical Labs Public Health Labs • Diagnostic testing • Some diagnostic testing • Some reference testing • Reference testing • Patient management • Surveillance and monitoring • Front line PH Response • Information to Clinical labs Individual health Public health Interdependent Network National Laboratory System Defining the State Laboratory System, 2010 Definition: “An alliance of laboratories and other partners within a state that supports the ten essential public health services under the aegis of the state public health laboratory. The system members and stakeholders operate in an interconnected and interdependent way to facilitate the exchange of information, optimize laboratory services, and help control and prevent disease and public health threats.” Building State Laboratory Systems for all Programs MLS Surveying Clinical Labs Establishing linkages Education Proficiency Testing MINNESOTA LABORATORY SYSTEM A PUBLIC AND PRIVATE COLLABORATION Ebola Funding: Enhanced Laboratory Biosafety and Biosecurity Capacity • To support public health departments and their clinical partners to assess, develop and implement measures to improve laboratory and biological safety practices for dealing with current and emerging infectious diseases. • Project Outcomes: – More trained staff knowledgeable in working with infectious organisms and other emerging pathogens of public health concern. – Improved biosafety practices for handling/processing Ebola and other highly infectious specimens at public health and clinical labs – Better coordination of biosafety practices between public health labs and clinical partners – Labs better equipped to serve jurisdiction in the inactivation and disposal Laboratory Efficiencies Initiative (LEI): CDC Director’s initiative (2011) to sustain PHL services “The LEI needs to accomplish what more than 100 individual labs cannot accomplish by working alone.” – Dr. Thomas Frieden Collective Solutions to Improve Effectiveness and Efficiency • • • • • • Regional networks for shared services Guidance for Policy and Practice Informatics capacity development Comprehensive data on PHL services PHL workforce development - Competencies Workflow efficiencies (Lean) Laboratory Integration Unit (LIU)/Division of Laboratory Systems • • Mission: To maximize the effectiveness and sustainability of state and local public health laboratory (PHL) testing services and capacity for surveillance and health protection nationally. Strategy: Promote coordinated CDC support for an integrated, national PHL system REGIONAL NETWORKS WORKFORCE DEVELOPMENT LABORATORY INFORMATICS COMPREHENSIVE DATA ON PHL TESTING SERVICES Fund Regional Networks/Consortia • Incentivize states to form consortia or regions to promote test sharing and other activities (training, data sharing) • Provides infrastructure, enabling multiple programmatic efforts to assure access to testing capacity in the states • Northeast Environmental and Public Health Laboratory Directors (NEEPHLD) (NY, NJ, NH, MA, RI, CT, others: ME, VT, New York City) • Northern Plains Consortium • 5 states (MT, ND, SD, WY, ID) Expected Outcomes • Sharing testing and training services, resources and strategies • Evidence/evaluation of cost effectiveness for interstate test referral • Logistical, legal, and financial requirements • Develop scalable solution for interstate electronic test order and results (ETOR) • Enabling robust mechanisms that are flexible to accommodate any testing OVERVIEW • Developed by informatics SMEs (CDC, PHLs) to assess capabilities across the entire informatics enterprise • The tool is intended for public health laboratories (PHLs) and is generalizable to clinical laboratories • Identify gaps in informatics capabilities through development and use of a ‘gold standard’ for comparison • Develop roadmap and steps for a PHL to benchmark and measure progress in its informatics capacity over time 19 CAPABILITY AREAS CA #1 CA #2 Laboratory Test Request and Sample CA #11 Contract and Grant Management receiving Test Preparation, LIMS Processing, Test Training, Education and Resource CA #12 Results Recording and Verification Management CA #3 Report Preparation and Distribution CA #13 Laboratory Certifications/Licensing CA #4 Laboratory Test Scheduling CA #14 Customer Relationship Management CA #5 Prescheduled Testing CA #15 CA #6 CA #7 Specimen and Sample Tracking/Chain of Custody Media, Reagents, Controls: Manufacturing and Inventory CA #16 CA #17 CA #8 Interoperability and Data Exchange CA #18 CA #9 Statistical Analysis and Surveillance CA #19 CA #10 Billing for Laboratory Services Quality Control (QC) and Quality Assurance (QA) Management Laboratory Safety and Accident Investigation Laboratory Mutual Assistance/Disaster Recovery Core IT Services: Hardware, Software and Services Policies and Procedures, including Budgeting and Funding SAMPLE CAPABILITY AREA DESIRED OUTCOMES – FOR PHLs • Provides a peer-reviewed metric to seek improvements • New Hampshire state PHL – Conducted the assessment and used results for the laboratory’s strategic plan and a capital budget request Copy of downloadable Access file available online: http://www.aphl.org/aphlprograms/lss/Laboratory-EfficienciesInitiative/Pages/Informatics.aspx Consolidating Test Service Data for PHL Directors Public Health Laboratory System Database (PHLSD) • Manage and control PHL’s own capacity data • Create a nationwide test service directory • Information easily used to meet the APHL survey and CDC data requests • Centralized location to find laboratory information Test Information by Organism PHLSD Linked to LOINC Codes Main Data Tab Personnel Data Tab Regulatory Data Tab Testing Data Tab Equipment Data Tab FEEDBACK “I wish I had this tool during last year’s hepatitis outbreak so I could easily know who to contact for assistance. A national test database would allow us to view methods and equipment utilized by other public health laboratories across the nation. This feature will be quite beneficial in surge events as contact information will also be included in the database.” -Dr. Christine Bean, Laboratory Director, New Hampshire Division of Public Health Services, Department of Health and Human Services “It was critical that Iowa could use the APHL Survey Resource Center (SRC) to find other state laboratories that could provide surge testing services for parasitology. The PHLSD management database will be an even greater improvement by allowing us to immediately identify states that provide the specific microscopy tests for Cyclospora and their testing capacity.” -Bonnie Rubin, Assoc. Director for Administrative Service, State Hygienic Laboratory at the University of Iowa Workforce: Public Health Laboratory* Competencies Laboratory workforce shortage is multifactorial Competencies are integral to any workforce development program, supporting job descriptions, performance objectives and evaluations, training and education programs, recruiting and orienting new staff, etc In 2012, APHL and CDC formed a competencies partnership o CSELS led o Involvement across CDC’s scientific CIOs– governance, SME input o Overall, >160 people worked to develop the competencies: CDC, APHL, state/local PHLs, state environmental lab, federal and state agriculture labs, clinical laboratories, academia * Broadly defined to include governmental public health, environmental, and agriculture labs Previous Collaboration: Biosafety Competencies The lack of defined workforce competencies was identified as a major gap in PHL workforce development Project Launch • CDC/APHL Steering Committee to support and guide project • Oct 2012 kick-off meeting – Guidance by competencies expert – Present overview of competencies – Working meeting to: Define project scope, target audience Prioritize stakeholder expectations Establish criteria for writing comps Determine comp structure, proficiency tiers Develop list of draft comp domains Workgroups and Teams • Establishment of Domain teams to develop competencies – Facilitated conference calls • Vetting – Incl clinical lab reps and others not involved in development – Adjudication Process workgroup • Lots of harmonization and quality control: – Various workgroups to standardize language, address gaps and overlaps, design the process for adjudicating comments Competency Structure and Rules for Writing Bloom’s Taxonomy Broad statements describing the “what”, not the “how” observable and measurable No correlation to any particular job title or degree! One verb only! modifiers Workgroups and Teams • Lots of harmonization and quality control – Development workgroup (strawman documents) – Synthesis workgroup (assess gaps/overlaps; harmonize language) – Harmonization of Domains workgroup and small teams – APHL and CDC project managers Timeline: March 2015 Additional quality control and polishing Vetting Aug. 2012 Schematic of Competency Domains for PHL Professionals Teams of subject matter experts developed general, cross-cutting technical, and specialized competencies, with a quality management system as the foundation of every activity. Example Competency (Draft) I. Competency Domain: Quality Management System Purpose statement: The competencies in Quality Management System (QMS) address the knowledge, skills, and abilities required for developing a laboratory’s culture of quality. The essential elements integrate operations, services, and infrastructure into a system that meets applicable regulatory standards, professional guidelines, and customer requirements for ensuring and maintaining quality and continually improving laboratory services. Introduction (excerpt): QMS is a systematic approach for ensuring the consistent quality of the tests performed, the products created, the data generated, and the results reported. Operating within a quality system meets the needs and requirements of public health laboratories as well as the expectations of partners, stakeholders, and users (internal and external customers)… Adhering to quality standards for laboratory operations helps laboratories generate consistent, reliable, and reproducible data and results… Personnel management • • • • Developing position descriptions Defining job requirements/performance expectations Establishing career ladders Assessing individual staff performance Example Biosafety Outreach Officer Position Description Training/Professional development • Performing training needs assessments • Designing training courses/curriculum, education programs • Refining professional development plans Competency Assessment Tool Competency Assessment Tool Prepublication Use of PHL Competencies Mapping recommended and required competencies for biosafety training in response to laboratory incidents Developing position descriptions for Bioinformatics specialists as part of Advanced Molecular Detection (AMD) Initiative Identifying recommended competencies for the new CDC Laboratory Leadership Service (LLS) Fellowship (modeled after Epidemic Intelligence Service-EIS) Organizational and system capacity Assessing organizational capacity Improving program development and management Supporting quality improvement Ensuring a sustainable PHL system Competency Infinity Cycle Infinity cycle courtesy of the International Food Protection Training Institute (IFPTI). Advocacy Articulating the role of PHLs in the public health system Standardization across the PHL system Promotion Summary • Public health and clinical laboratories form networks to support public health • Sustainability is improved through shared solutions and tools • The PHL competencies provide value to personnel management, training/professional development, and organizational and system capacity • The PHL competencies are universal for laboratory practice For more information please contact: John Ridderhof, DrPH Senior Advisor for Laboratory Integration (OPHSS/CSELS) THANK YOU The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.