Chemical Sedimentary Rocks.
advertisement

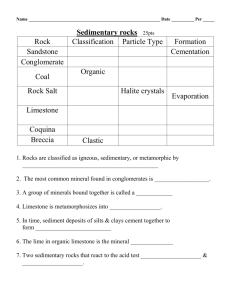
CHEMICAL SEDIMENTARY ROCKS Prepared by Dr. F. Clark Department of Earth and Atmospheric Sciences, University of Alberta August 06 INTRODUCTION Chemical sedimentary rocks would be those whose grains formed by direct precipitation or crystallization from water, without organic influence or mediation. This latter qualification is somewhat problematic, given that most bodies of water have organisms which will influence the chemistry of that water at least to some extent. There is one volumetrically and economically significant group of sedimentary rocks of non-controversial chemical origin, the so-called evaporites. As their name implies, they form by evaporation of concentrated sea or saline lake water. EVAPORITES As a body of sea water or a saline lake experiences net evaporation, the concentration of the ions dissolved in that water rises until the saturation point of various materials is exceeded, and minerals precipitate or crystallize. Many of these minerals are economically significant, such as gypsum, halite, and potash salts from sea water, and epsom salts, borax and trona from saline lakes. The first minerals to form as the water evaporates are carbonates, which we have covered already under biochemical sedimentary rocks. They are generally volumetrically minor components of evaporite mineral assemblages. Gypsum. This is hydrated calcium sulphate; the sulphates are the second major group to form as sea water evaporates. Such large crystals as these are frequently formed by precipitation from saturated groundwater circulating through near-surface sediment deposits and soils, rather than precipitation from sea water. Note the clarity of these large crystals, which have a Mohs hardness of 2. Gypsum – the Effect of Crystal Size Both photos illustrate the effect on opacity that crystal size has. These evaporite samples consist of thousands of individual small crystals, whose edges and grain boundaries dominate the optical effects and render the samples opaque, even though gypsum is transparent to translucent. The right sample is from the Devonian age Elk Point Group of the Western Canada Sedimentary Basin. Anhydrite. This is calcium sulphate without the bound molecular water that defines gypsum. In this sample it is white. This is less likely to form as a primary evaporite mineral, because the presence of water makes gypsum formation more likely. It is possible to dehydrate gypsum after its initial formation, or as apparently happened in this case, for anhydrite to form in a carbonate host rock. Halite – Rock Salt, NaCl Halite forms third in the sequence of evaporation of sea water, and in a closed system would account for approximately 75% of all the solids that will form. It may be mined conventionally, or as suggested by the core sample on the right, can be recovered from bore holes by circulation of water in the subsurface and evaporation of the resulting brines at surface. The salty taste is distinctive. Sylvite. This is potassium chloride (KCl), and unlike halite, has a distinctly bitter taste. Its other properties are similar to those of halite. The fourth group to form from the evaporation of sea water is a complex assemblage, whose most useful member is sylvite. It is found in the potash deposits of Saskatchewan, and is a major component in fertilizer production. It forms when 98% of the water is lost. CHERT – PROBLEMATIC ORIGIN Chert, a microcrystalline or cryptocrystalline form of silica (SiO2), can originate either by organic means or not. There are two principal groups of organisms that secrete siliceous skeletons whose accumulation can result in the formation of bedded chert. Radiolarians are microscopic plankton that are less than 1 mm in size. As well, there are some sponges whose spicules (tiny hard parts that support the tissues) are siliceous. These groups first appeared in the Cambrian, so older rocks (>544 million years old) hypothetically can’t be biochemical. Even younger chert may be chemical in origin, forming during diagenesis as nodules and beds, usually in carbonates. Chert. Chert has many properties in common with quartz. It has a hardness of 7, and samples exhibit conchoidal fracture. There are many coloured varieties of chert, ranging from white through yellow and pale green to black, depending on trace elements and their chemical state (e.g. red for oxidized and green for reduced iron). Chert from Western Canada The occurrence of chert as beds or bands, especially in carbonate sections, is quite common in the rocks of the Western Canada Sedimentary Basin. From left to right we see examples from the Mississippian Rundle Group, the Pennsylvanian Kananaskis Formation, and the Permian Ishbel Group. Not only is chert common in these rocks, but when they are weathered after uplift during building of the Rocky Mountains, they form a rich source of the common chert pebbles and sand grains seen in Cretaceous age siliciclastic units.