Solubility of KNO3 - SCED360
advertisement

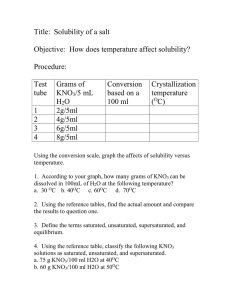
Tagandurdy Berdiyew 2004000125 Solubility of KNO3 With this experiment our purpose was to find the solubility of KNO3 in 100 ml water. Firstly, we weighed 1, 2, 4 and 6 grams of KNO3 samples and transferred them into 4 test tubes. Then these tubes filled with 5ml of distilled water. Then we heated them in water bath until we obtain clear solution. Then, we had taken our dissolved solutions and started to cool them at room temperature until we observe first precipitated crystals since the first crystallization step is the maximum solubility of KNO3 in 5 ml water at a specific temperature. We firstly observed crystallization of 6 g of KNO3 sample at 58 °C. Finally, we get the following data: Test Mass Volume Convert to Saturation Tube KNO3(g) Water (ml) g/100 ml Temp (°C) 1 1 5 20 16 2 2 5 40 24 3 4 5 80 48 4 6 5 120 58 Finally, we had drawn a dissolved gr of KNO3/100 ml H2O vs. temperature graph by using the obtained data. Thus, we obtained the approximate solubility of KNO3 in 100 ml water at a specific temperature. By this experiment we can teach saturated, unsaturated, super saturated, concentrated and dilute solutions concepts.