PS 1c
advertisement

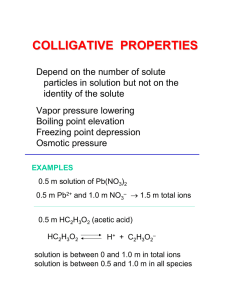
Problem Set 1c Chem 142 Show all work (where appropriate) for full credit. If you need a value for a constant, look it up. 1. In each group, pick the one that has the property listed: (a) highest freezing point: 0.1 M KI (aq), (b) highest boiling point: 0.2 M C6H12O6 (aq), (c) most volatile: H2O (d) greatest osmotic pressure: 0.1 M NaOH (aq) (e) lowest vapor pressure: 2. 0.1 M NH2OH (aq), H2O 0.1 M CaCl2 (aq) 0.2 M NH4F (aq) 0.2 M BaCl2 (aq) 0.1 M NaNO3 (aq) 0.1 M CH3OCH3 (aq) 0.1 M HOCH2CH2OH (aq) 0.05 M NH4NO3 (aq) H2O 0.05 M CaBr2 (aq) Methyl alcohol (methanol, CH3OH) and ethylene glycol (HOCH2CH2OH) are both used to prevent the freezing of water in automobile radiators during cold weather. (Hint: this is more conceptual than quantitative. Assume the solutes are non-volatile) You must explain why for each. (a) Which will be more effective in a given radiator, an aqueous solution containing 25 g of methanol or 25 g of ethylene glycol dissolved in water? (b) Which will be more effective, a 5 m solution of methyl alcohol or 5 m solution of ethylene glycol? . over Chem 142, Dr. Haefner 3. The molal freezing point constant for copper is 23.0 °C kg/mol. If pure copper melts at 1083 °C, what will be the melting point of a brass made of 10.0 mass % Zn and 90.0 mass % Cu? 4. A 1.08 g sample of the blood plasma protein, human serum albumin, is dissolved in enough H2O to bring the solution volume to 20.0 mL. The resulting solution had an osmotic pressure of 5.85 mm Hg at 298 K. What is the molar mass of albumin? over Chem 142, Dr. Haefner 5. What is the vapor pressure of a 3.0 M solution of C6H12O6 (aq) (MW = 180.16 g/mol, solution density = 1.12 g/ml) at 100C? Assume that C6H12O6 is non-volatile . 6. What would be the vapor pressure for a 3.0 M Na2SO4 (aq) solution (assume the same solution density as in 5) **hint: consider how the differences between C6H12O6 and Na2SO4 change the calculation** 7. What is the boiling point of the solution in problem 5? over