document

Overview Lecture:
Carboxylic Acids, Esters, Amines
& Amides
Last Lecture Overview 1
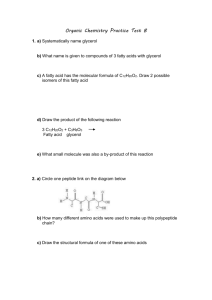
Carboxylic Acids
O have the structure R-C-O-H , also written as:
RCOOH and RCO
2
H.
-COOH is called the carboxyl group.
Last Lecture Overview 2
Nomenclature of RCOOH :
1. Find the longest chain that includes COOH.
2. Parent name becomes alkane + oic acid.
3. C of COOH becomes #1.
4. COOH more important than other functional groups.
Last Lecture Overview 3
Example
3
O
CH
3
-CH
2
-CH-CH
5 4
OH
2
2
-COH
1
3-hydroxy pentanoic acid
Last Lecture Overview 4
Carboxylic Acids are weak acids:
They are H + donors, but not completely ionized in water.
H-A H + + A
–
K a
<< 1, which means that
At equilibrium mostly HA present.
Last Lecture Overview 5
Amines are organic bases derived from ammonia (NH
3
).
In amines a carbon group replaces an H in NH
3
.
Last Lecture Overview 6
Ammonia = H-N-H
H
R -NH
2
1 ° Amine
R -NHR’
2 ° Amine
Last Lecture Overview
R -NR ’
R ”
3 ° Amine
7
Simple Naming of Amines
Name the alkyl groups bonded to
N, followed by the word “amine” :
CH
3
CH
2
-NH-CH
3 is named ethyl methyl amine
Last Lecture Overview 8
Amines
* are Hydrogen-bond donors and
H-bond acceptors
* are weak bases
Last Lecture Overview 9
Amines are bases because they are
H + acceptors.
All amines are weak bases like NH
3
.
R-NH
2
+ H
2
O R-NH
3
+ + HO -
K << 1
Last Lecture Overview 10
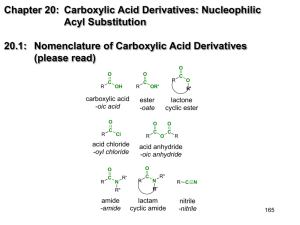
Esters and Amides are obtained by replacing the OH group of RCOOH.
O
R-COH
O
R-COR’
Ester
O
R-CN
Amide
R’
R”
Last Lecture Overview 11
The OH of RCOOH can be replaced chemically in several steps in the lab or by enzymes.
More important than how:
1) Esters come from RCOOH + ROH
(an alcohol); while
2) Amides come from RCOOH +
Amine or Ammonia.
Last Lecture Overview 12
Example of Ester Synthesis
O CH
3
CH
3
CH
2
C OH + H O-CH
2
CHCH
3
- H
2
O by enzyme or in lab
O CH
3
CH
3
CH
2
C O-CH
2
CHCH
3
Last Lecture Overview 13
Thus, the basic equation is
Carboxylic acid + alcohol ester + water
Last Lecture Overview 14
Example of Amide Synthesis
O
CH
3
CH
2
C OH + H NH-CH
3
- H
2
O by enzyme or in lab
O
CH
3
CH
2
C NH-CH
3
Last Lecture Overview 15
Again, the basic equation is
Carboxylic acid + amine or NH
3 amide + water
Last Lecture Overview 16
Esters and Amides are hydrolyzed
(broken down by H
2
O in H + or HO ).
Ester + H
2
O Carboxylic acid + Alcohol
Amide + H
2
O Carboxylic acid + Amine
(or ammonia)
Last Lecture Overview 17
O
Hydrolysis Examples
O
Ester
C-OCH
2
CH
2
CH
3
H
2
O with enzyme or H + or HO -
COH + HOCH
2
CH
2
CH
3 carboxylic acid alcohol
Last Lecture Overview 18
Hydrolysis Example 2
O
CH
3
C-NHAmide
H
2
O with enzyme or H + or HO -
O
CH
3
C-OH + H
2
Ncarboxylic acid amine
Last Lecture Overview 19
That’s All Folks
The End
Last Lecture Overview 20