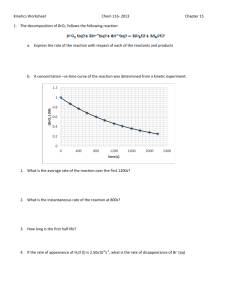
APC-Ch.12-Chemical Kinetics
advertisement

Chemical Kinetics Chapter 12 Unit 7 - Chemical Kinetics AP Chemistry Reaction Rates Factors that Affect Reaction Rates 1.The physical state of the reactants Reaction is limited to area of contact when in different phases ✦ Increase surface area are of solids to increase rate 2.The concentration of the reactants ✦ For most, as concentration increases, rate increases 3.The temperature at which the reaction occurs ✦ As temperature increases, the rate increase ✦ Reaction Rates Factors that Affect Reaction Rates 4.The presence of a catalyst 5.On a molecular level, depends on the frequency of collisions. ✦ As collisions increase, the rate increases ✦ Collisions must occur with sufficient energy and with correct orientation Reaction Rates Reaction Rates ✦ A rate is change over time concentration of A at time t 2 - concentration of A at time t1 Rate = t 2 - t1 ✦ Δ [ A] = Δt units are M/s Reaction Rates Reaction Rates Sample Problem A Consider the following reaction N 2 ( g ) + 3H 2 ( g ) ⎯⎯ → 2NH 3 ( g ) What happens to each of the reactants as the reaction progresses? Reaction Rates Reaction Rates Sample Problem A As the reaction progresses, the concentration of H2 goes down. Concentration ✦ [H2] Time Reaction Rates Reaction Rates Sample Problem A As the reaction progresses, the concentration of N2 goes down 1/3 as fast. Concentration ✦ [N2] [H2] Time Reaction Rates Reaction Rates Sample Problem A As the reaction progresses, the concentration of NH3 goes up. Concentration ✦ [N2] [H2] [NH3] Time Reaction Rates Reaction Rates and Stoichiometry ✦ ✦ ✦ In general, for a reaction aA + bB ⎯⎯ → cC + dD The rate is given by 1 Δ [ A] 1 Δ [ B] 1 Δ [C ] 1 Δ[ D] Rate = − =− =− =− a Δt b Δt c Δt d Δt So for the previous reaction, what does the rate equal? Rate = − Δ [ N2 ] Δt 1 Δ[ H2 ] 1 Δ [ NH 3 ] =− =− 3 Δt 2 Δt Reaction Rates Reaction Rates and Stoichiometry Sample Problem B a.How is the rate at which ozone disappears related to the rate at which oxygen appears in the reaction 2O3 ( g ) ⎯⎯ → 3O2 ( g ) -5 b.If the rate at which O2 appears, Δ[O2]/Δt, is 6.0 x 10 M/s at a particular instant, at what rate is O3 disappearing at this same time, Δ[O3]/Δt? Reaction Rates Change of Rate with Time ✦ ✦ Average rates are taken over long intervals Instantaneous rates are determined by finding the slope of a line tangent to the curve at any given point because the rate can change over time. Concentration Average Slope Method D[H2] Dt Time Concentration Instantaneous Slope Method Reaction rates are not constant if the reaction depends on concentration Δ[ H2 ] Δ (t ) Time Rate Laws Types of Rate Laws 1.Differential Rate Law - looks at the effect of concentration on the reaction rate. ✦ Usually just referred to as the rate law ✦ Must come from a set of data 2.Integrated Rate Law - looks at how the concentration of reactants change over time Rate Laws Forming the Rate Law aA + bB ⎯⎯ → cC + dD Rate = k [ A ] [ B ] k = rate constant (units vary) m, n = reaction orders (usually small whole numbers) [ ] = concentration of reactants m + n = overall reaction order m ✦ ✦ ✦ ✦ n Rate Laws Forming the Rate Law Example Rate = k ⎡⎣ NH ⎤⎦ ⎡⎣ NO ⎤⎦ 1+ 4 1− 2 m = 1, n = 1 ✦ we say the reaction is 1st order in NH41+ or 1st order in NO21-. ✦ The reaction is 2nd order overall. ✦ This means that because the rate at which NH41+ reacts with 11+ NO2 depends on [NH4 ] raised to the 1st power, the rate 1+ doubles when [NH4 ] doubles (triples when tripled, etc...). And 1the same goes for [NO2 ]. ✦ Rate Laws Forming the Rate Law Example Rate = k ⎡⎣ NH ⎤⎦ ⎡⎣ NO ⎤⎦ If a rate law is 2nd order with respect to a reaction, [A]2, doubling the concentration of that substance will quadruple the reaction rate. The values of the exponents are NOT determined by the balanced equation. They are determined experimentally! 1+ 4 ✦ ✦ 2N 2O5 ⎯⎯ → 4NO2 + O2 H 2 + I 2 ⎯⎯ → 2HI CHCl3 + Cl2 ⎯⎯ → CCl4 + HCl 1− 2 Rate = k [ N 2O5 ] Rate = k [ H 2 ][ I 2 ] Rate = k [ CHCl3 ][ Cl2 ] 1 2 Rate Laws Forming the Rate Law ✦ ✦ The RATE of a reaction depends on concentration, but the RATE CONSTANT does not. The rate constant is only affected by the temperature change and the presence of a catalyst. Rate Laws Determining Rate Law Sample Problem C The initial rate of a reaction A + B → C was measured for several different starting concentrations. Using the datas below, find a.the rate law b.the rate constant c.the rate of reaction when [A] = 0.500 M and [B] = 0.100 M Experiment 1 2 3 [A] (M) 0.1 0.1 0.2 [B] (M) 0.1 0.2 0.1 Initial Rate (M/s) -5 4.0 x 10 -5 4.0 x 10 -5 16.0 x 10 Rate Laws Determining Rate Law Sample Problem D 2NO + 2H 2 ⎯⎯ → N 2 + 2H 2O Experiment 1 2 3 [NO] (M) 0.1 0.1 0.2 [H2] (M) 0.1 0.2 0.1 Initial Rate (M/s) -3 1.23 x 10 -3 2.46 x 10 -3 4.92 x 10 a.Find the rate law. b.Find the rate constant (with units). c.Calculate the rate when [NO] = 0.050 M and [H2] = 0.150 M Rate Laws Determining Rate Law Sample Problem E Rate Data for the Reaction of Ammonia and Nitrate Ions in Water Experiment [NH41+] (M) [NO21-] (M) Initial Rate (M/s) 1 0.01 0.2 5.4 x 10-7 2 0.02 0.2 10.8 x 10-7 3 0.04 0.2 21.5 x 10-7 4 0.06 0.2 32.2 x 10-7 5 0.2 0.0202 10.8 x 10-7 6 0.2 0.0404 21.5 x 10-7 7 0.2 0.0606 32.2 x 10-7 8 0.2 0.0808 43.3 x 10-7 a.Determine the rate law. b.Calculate the rate constant. Rate Laws Change of Concentration with Time (Integrated Rate Law) ✦ ✦ Converts the rate law into an equation that tells what the concentration is at a particular point in time. If you memorize and can use the following table, you will know 90% of the kinetics information on the AP Exam! Rate Laws Kinetics Table Order Rate Law Integrated Rate Law Straight Line Plot Rate Constant and Slope Half-Life Zeroth First Second Rate = k Rate = k [ A ] Rate = k [ A ] ln [ A ] = −kt + ln [ A ]0 1 1 = kt + [ A] [ A ]0 [ A ] vs t ln [ A ] vs t 1 vs t [ A] slope = −k slope = −k slope = k 0.693 t 12 = k 1 t 12 = k [ A ]0 [ A ] = −kt + [ A ]0 t 12 A ]0 [ = 2k 2 Rate Laws AP Exam Practice Sample Problem F Answer the following questions related to the kinetics of chemical reactions. I − → IO ( aq ) + Cl ( aq ) ( aq ) + ClO ( aq ) ⎯⎯⎯ I, − OH − − − IO , Iodide ion, is oxidized by hypoiodite ion, by hypochlorite, ClO , in basic solution according to the equation above. Three initial-rate experiments were conducted; the results are shown in the following table. Rate Laws AP Exam Practice Sample Problem F Experiment 1 2 3 [I ] (mol/L) 0.017 0.052 0.016 [ClO ] (mol/L) 0.015 0.015 0.061 Initial Rate of Formation of (mol/L*s) 0.156 0.476 0.596 IO a.Determine the order of the reaction with respect to each reactant listed below. Show your work. i. I (aq) ii.ClO (aq) Rate Laws AP Exam Practice Sample Problem F Experiment 1 2 3 [I ] (mol/L) 0.017 0.052 0.016 [ClO ] (mol/L) 0.015 0.015 0.061 Initial Rate of Formation of (mol/L*s) 0.156 0.476 0.596 IO b.For the reaction, i. write the rate law that is consistent with the calculations in part (a); ii.calculate the value of the specific rate constant, k, and specify units. Rate Laws AP Exam Practice Sample Problem F The catalyzed decomposition of hydrogen peroxide, H2O2(aq), is represented by the following equation. catalyst 2H 2O 2 ( aq ) ⎯⎯⎯→ 2H 2O ( l ) + O2 ( g ) The kinetics of the decomposition reaction were studied and the analysis of the results show that it is a first-order reaction. Some of the experimental data are shown in the table below. [H2O2] Time (min) 1 0 0.78 5 0.61 10 AP* Chemistry: Kinetics Rate Laws Name: ______________________________ Period:_____________ AP Exam Practice (c) During the analysis of the data, the graph below was produced. Sample Problem F c.During the analysis of the data, the graph below was produced. i. Label the vertical axis of the graph. (i) Label the vertical axis of the graph that is repeated on your student answer pages. ii.What are the units of the rate constant, k, for the decomposition (ii) What are the units of the rate constant, k , for the decomposition of H O (aq) ? of H2O2(aq)? (iii) On the graph repeated on your student answer pages, draw the line that represents the plot of the uncatalyzed decomposition of 1.00 O (aq)of . the uncatalyzed firstiii.Draw the line first-order that represents theM Hplot order decomposition of 1.00 M H2O2(aq). 2 2 2 NO CALCULATORS MAY BE USED 2 Rate Laws Half-Life Sample Problem G The decomposition of a certain insecticide in water follows 1st order -1 kinetics with a rate constant of 1.45 yr . A quantity of the insecticide is washed into a lake on June 1, leading to a -7 3 concentration of 5.0 x 10 g/cm . a.What is the concentration of insecticide on June 1 of the following year? b.How long will it take for the concentration of the insecticide to -7 3 drop to 3.0 x 10 g/cm ? A Model for Chemical Kinetics Activation Energy A Model for Chemical Kinetics Endothermic vs. Exothermic A Model for Chemical Kinetics Determining Activation Energy ✦ ✦ Use Arrhenius equation Ea ln k = − + ln A RT Graphically the equation shows that a graph of ln k vs. 1/T will have the slope equal to -Ea/R with a y-intercept at ln A. Reaction Mechanisms Reaction Mechanisms ✦ ✦ A balanced chemical reaction only shows what happens at the beginning and the end. Mechanism shows what happens at all points during the reaction by showing the elementary steps in the reaction. ✦ Each step should add together to form the balanced chemical equation. ✦ The mechanism must agree with the given rate law. Reaction Mechanisms Rate Determining Step ✦ ✦ In a multi-step mechanism, the slowest step is the rate-determining step. The rate-determining step therefore determines the rate of the reaction. The experimental rate law must agree with the rate determining step. Reaction Mechanisms Rate Determining Step Sample Problem H 2NO ( g ) + Br2 ( g ) ⎯⎯ → 2NOBr ( g ) a.Identify the rate determining step. Step 1 NO ( g ) + Br2 ( g ) ←⎯ → NOBr2 ( g ) Step 2 NOBr2 ( g ) + NO ( g ) ⎯⎯ → 2NOBr ( g ) b.Identify the rate law. c.Identify any intermediates. (slow ) ( fast ) Catalysts Catalysts ✦ Catalyst - a substance that speeds up a reaction without being consumed (this does not mean that it can be changed during the process) ✦ lowers activation energy ✦ increases the number of collisions ✦ Homogeneous catalyst - in the same phase as the reactants ✦ Heterogeneous catalyst - in a different phase ✦ Enzyme - type of biological catalyst Rate Laws AP Exam Practice Sample Problem I An environmental concern is the depletion of the O3 in Earth’s upper atmosphere, where O3 is normally in equilibrium with O2 and O. A proposed mechanism for the depletion of O3 in the upper atmosphere is shown below. Step I O3 + Cl ⎯⎯ → O2 + ClO Step II ClO + O ⎯⎯ → Cl + O2 a.Write a balanced equation for the overall reaction represented by Step I and Step II above. Rate Laws AP Exam Practice Sample Problem I An environmental concern is the depletion of the O3 in Earth’s upper atmosphere, where O3 is normally in equilibrium with O2 and O. A proposed mechanism for the depletion of O3 in the upper atmosphere is shown below. Step I O3 + Cl ⎯⎯ → O2 + ClO Step II ClO + O ⎯⎯ → Cl + O2 b.Clearly identify the catalyst in the mechanism above. Justify your answer. Rate Laws AP Exam Practice Sample Problem I An environmental concern is the depletion of the O3 in Earth’s upper atmosphere, where O3 is normally in equilibrium with O2 and O. A proposed mechanism for the depletion of O3 in the upper atmosphere is shown below. Step I O3 + Cl ⎯⎯ → O2 + ClO Step II ClO + O ⎯⎯ → Cl + O2 c.Clearly identify the intermediate in the mechanism above. Justify your answer. Rate Laws AP Exam Practice Sample Problem I Step I O3 + Cl ⎯⎯ → O2 + ClO Step II ClO + O ⎯⎯ → Cl + O2 d.If the rate law for the overall reaction is found to be rate=k[O3][Cl], determine the following. i. The overall order of the reaction. ii.Appropriate units for the rate constant, k iii.The rate-determining step of the reaction, along with justification for your answer. Daily Objectives Chemistry II Honors Today, I will be able to use kinetic experiment data to calculate the rate law, rate law constant, and the units of the rate law constant. • LO 4.1, 4.2, 4.3, 4.4, 4.5, 4.6, 4.7, 4.8, 4.9 Rate Laws AP Exam Practice Sample Problem J Consider the following general equation for a chemical reaction. 0 A ( g ) + B ( g ) ⎯⎯ → C ( g) + D ( g) ΔH rxn = −10 kJ a.Describe the two factors that determine whether a collision between molecules of A and B results in a reaction. b.How would a decrease in temperature affect the rate of reaction shown above? Explain your answer. Rate Laws AP Exam Practice Sample Problem J Consider the following general equation for a chemical reaction. 0 A ( g ) + B ( g ) ⎯⎯ → C ( g) + D ( g) ΔH rxn = −10 kJ c.Write the rate law expression that would result if the reaction proceeded by the mechanism shown below. A + B ⎯⎯ → [ AB ] ( fast ) →C + D [ AB ] + B ⎯⎯ (slow ) d.Explain why a catalyst increases the rate of a reaction but does not change the value of the equilibrium constant for that reaction. Rate Laws AP Exam Practice Sample Problem K A ( aq ) + 2B ( aq ) ⎯⎯ → 3C ( aq ) + D ( aq ) For the reaction above, carried out in a solution of 30ºC, the following kinetic data were obtained: Experiment 1 Initial Conc. of Reactants Initial Rate of Reaction A0 B0 0.24 0.48 8 2 0.24 0.12 2 3 0.36 0.24 9 4 0.12 0.12 0.5 5 0.24 0.06 1 6 0.014 1.35 ? Rate Laws AP Exam Practice Sample Problem K A ( aq ) + 2B ( aq ) ⎯⎯ → 3C ( aq ) + D ( aq ) Experiment 1 Initial Conc. of Reactants Initial Rate of Reaction A0 B0 0.24 0.48 8 2 0.24 0.12 2 3 0.36 0.24 9 4 0.12 0.12 0.5 5 0.24 0.06 1 6 0.014 1.35 ? a.Write the rate-law expression for this reaction. b.Calculate the value of the specific rate constant k at 30ºC and specify its units. Rate Laws AP Exam Practice Sample Problem K A ( aq ) + 2B ( aq ) ⎯⎯ → 3C ( aq ) + D ( aq ) Experiment 1 Initial Conc. of Reactants Initial Rate of Reaction A0 B0 0.24 0.48 8 2 0.24 0.12 2 3 0.36 0.24 9 4 0.12 0.12 0.5 5 0.24 0.06 1 6 0.014 1.35 ? c.Calculate the value of the initial rate of this reaction at 30ºC for the initial concentrations shown in experiment 6. d.Assume that the reaction goes to completion. Under the conditions specified for experiment 2, what would be the final molar concentration of C? Rate Laws AP Exam Practice Sample Problem L 2ClO2 ( g ) + F2 ( g ) ⎯⎯ → 2ClO2 F ( g ) The following results were obtained when the reaction represented above was studied at 25ºC. Experiment Initial Conc. of Reactants Initial Rate of Reaction of [ClO2] [F2] [ClO2F] 1 0.01 0.1 2.4 x 10-3 2 0.01 0.4 9.6 x 10-3 3 0.02 0.2 9.6 x 10-3 Rate Laws AP Exam Practice Sample Problem L 2ClO2 ( g ) + F2 ( g ) ⎯⎯ → 2ClO2 F ( g ) Experiment Initial Conc. of Reactants Initial Rate of Reaction of [ClO2] [F2] [ClO2F] 1 0.01 0.1 2.4 x 10-3 2 0.01 0.4 9.6 x 10-3 3 0.02 0.2 9.6 x 10-3 a.Write the rate law expression for the reaction above. b.Calculate the numerical value of the rate constant and specify the units. Rate Laws AP Exam Practice Sample Problem L 2ClO2 ( g ) + F2 ( g ) ⎯⎯ → 2ClO2 F ( g ) Experiment Initial Conc. of Reactants Initial Rate of Reaction of [ClO2] [F2] [ClO2F] 1 0.01 0.1 2.4 x 10-3 2 0.01 0.4 9.6 x 10-3 3 0.02 0.2 9.6 x 10-3 c.In experiment 2, what is the initial rate of decrease of [F2]? Rate Laws AP Exam Practice Sample Problem L d.Which of the following reaction mechanisms is consistent with the rate law developed in (a). Justify your answer. I. ⎯⎯ ⎯ → ClO2 + F2 ← ⎯ ClO2 F2 ClO2 F2 ⎯⎯ → ClO2 F + F ClO2 + F ⎯⎯ → ClO2 F ⎯⎯ ⎯ → II. F2 ← ⎯ 2F ( 2 ClO2 + F ⎯⎯ → ClO2 F ) ( fast ) ( slow ) ( fast ) ( slow ) ( fast ) Rate Laws AP Exam Practice Sample Problem M 2H 2O2 ( aq ) ⎯⎯ → 2H 2O ( l ) + O2 ( g ) Hydrogen peroxide decomposes according to the equation above. a.An aqueous solution of H2O2 that is 6.00 percent H2O2 by mass has a density of 1.03 g/mL. Calculate each of the following. i. The original number of moles of H2O2 in a 125 mL sample of the 6.00 percent H2O2 solution. ii.The number of moles of O2(g) that are produced when all of the H2O2 in the 125 mL sample decomposes. 2 H2O2(aq) 2 H2O(l) + O2(g) Hydrogen peroxide decomposes according to the equation above. Rate Laws (a) An aqueous solution of H2O2 that is 6.00 percent H2O2 by mass has a density of 1.03 g mL–1. Calculate each of the following. (i) The original number of moles of H2O2 in a 125 mL sample of the 6.00 percent H2O2 solution (ii) The number of moles of O2(g) that are produced when all of the H2O2in the 125 mL sample AP Exam Practice decomposes Sample Problem M (b) The graphs below show results from a study of the decomposition of H O . 2 2 b.The graphs below show results from a study of the decomposition of H2O2. i. Write the rate law for the reaction. Justify your answer. (i) Write the rate law for the reaction. Justify your answer. (ii) Determine the half-life of the reaction. ii.Determine the half-life of the reaction. (iii) Calculate the value of the rate constant, k. Include appropriate units in your answer. (iv) Determine [H2O2] after 2,000 minutes elapse from the time the reaction began. 2 H2O2(aq) 2 H2O(l) + O2(g) Hydrogen peroxide decomposes according to the equation above. Rate Laws (a) An aqueous solution of H2O2 that is 6.00 percent H2O2 by mass has a density of 1.03 g mL–1. Calculate each of the following. (i) The original number of moles of H2O2 in a 125 mL sample of the 6.00 percent H2O2 solution (ii) The number of moles of O2(g) that are produced when all of the H2O2in the 125 mL sample AP Exam Practice decomposes Sample Problem M (b) The graphs below show results from a study of the decomposition of H O . 2 2 b.The graphs below show results from a study of the decomposition of H2O2. iii.Calculate the value of the rate constant, k. Include appropriate (i) Write the rate law for the reaction. Justify your answer. units in your answer. (ii) Determine the half-life of the reaction. (iii) Calculate the value of the rate constant, k. Include appropriate units in your answer. (iv) Determine [H 2 O]] after 2,000 minutes elapse from the time the reaction began. iv.Determine [H2O after 2000 minutes elapse from the time the reaction begun. 2 2