Chemistry – Unit 5 Worksheet 1
advertisement
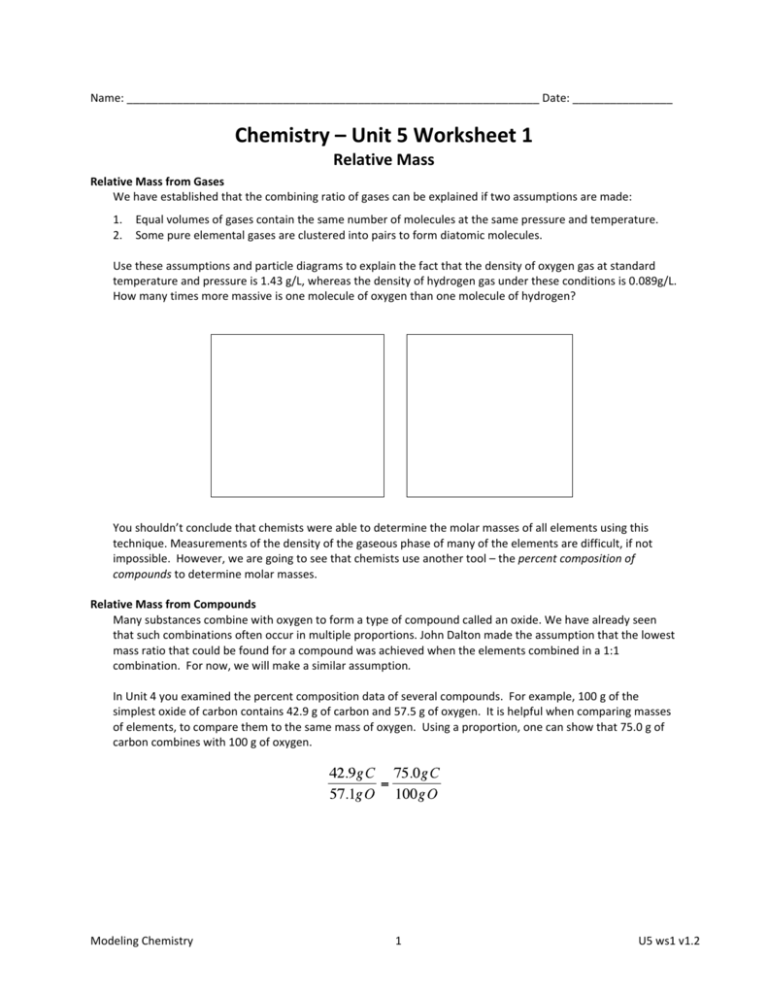
Name: __________________________________________________________________ Date: ________________ Chemistry – Unit 5 Worksheet 1 Relative Mass Relative Mass from Gases We have established that the combining ratio of gases can be explained if two assumptions are made: 1. 2. Equal volumes of gases contain the same number of molecules at the same pressure and temperature. Some pure elemental gases are clustered into pairs to form diatomic molecules. Use these assumptions and particle diagrams to explain the fact that the density of oxygen gas at standard temperature and pressure is 1.43 g/L, whereas the density of hydrogen gas under these conditions is 0.089g/L. How many times more massive is one molecule of oxygen than one molecule of hydrogen? You shouldn’t conclude that chemists were able to determine the molar masses of all elements using this technique. Measurements of the density of the gaseous phase of many of the elements are difficult, if not impossible. However, we are going to see that chemists use another tool – the percent composition of compounds to determine molar masses. Relative Mass from Compounds Many substances combine with oxygen to form a type of compound called an oxide. We have already seen that such combinations often occur in multiple proportions. John Dalton made the assumption that the lowest mass ratio that could be found for a compound was achieved when the elements combined in a 1:1 combination. For now, we will make a similar assumption. In Unit 4 you examined the percent composition data of several compounds. For example, 100 g of the simplest oxide of carbon contains 42.9 g of carbon and 57.5 g of oxygen. It is helpful when comparing masses of elements, to compare them to the same mass of oxygen. Using a proportion, one can show that 75.0 g of carbon combines with 100 g of oxygen. Modeling Chemistry 1 U5 ws1 v1.2 In like fashion, the masses of the elements in various oxides were calculated and shown in the table below. Element Mass in grams of element that would combine with 100g of oxygen to form simplest oxide Dalton’s Relative Mass compared to hydrogen Molar mass from periodic table Adjusted Relative Mass hydrogen 12.5 1.0 carbon 75.0 nitrogen 87.5 oxygen 100 iron 349 mercury 1250 silver 1349 1. If the elements in these compounds combine in a 1:1 ratio, the values in the 2nd column could be used to compare the masses of these elements to one another. In order to calculate masses relative to hydrogen, divide these values by the mass of hydrogen to obtain relative masses of the elements. Record these values in the 3rd column. These are the values Dalton reported for the relative masses of these elements. 2. Compare the relative mass of oxygen that you just placed in the table with the relative mass you determined using the density information from the front of the paper. 3. Look up the molar masses from the periodic table and fill them in to the 4th column. How do they compare to Dalton’s relative masses? Modeling Chemistry 2 U5 ws1 v1.2 4. Dalton assumed that in water, hydrogen and oxygen combined in a 1:1 ratio. We have seen that data shows us that hydrogen and oxygen actually combine in a 2:1 ratio. a. Sketch a particle diagram for Dalton’s view of water and a particle diagram for our modern view of water. b. Use your particle diagrams to adjust Dalton’s relative masses and record the adjusted relative masses in the 5th column. Show you reasoning for the adjustment below. 5. From the data in the table, are there any elements that do not combine with oxygen in a 1:1 ratio? Explain. Modeling Chemistry 3 U5 ws1 v1.2