Drawing Bohr-Rutherford Diagrams
advertisement

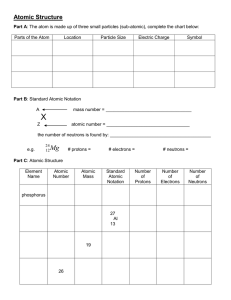
SNC 1D – Chemistry note Date: ____________ Drawing Bohr-Rutherford Diagrams To draw a Bohr-Rutherford atom you need to be able to find three things: the number or protons, number of electrons and the number of neutrons. If we know how they all fit together we can draw the diagrams. 1. Find the number of protons (atomic number = # of protons) 2. Find the number of electrons (# of protons = # of electrons it’s a neutral atom) 3. Calculate the number of neutrons (#neutrons = mass number – atomic number) 4. Draw a circle and write the number of each, protons and neutrons, inside the circle representing the nucleus. 5. Draw shells and fill will the correct number of electrons placed in pairs. (shell 1 = 2 electrons, shell 2 = 8 electrons, shell 3 = 8 electrons) (The electrons always fall to lowest possible energy level so fill lower shells first) Draw these atoms a b-r diagrams Na Cl He An _______________ is an atom with the same number of protons but different number of neutrons. Hydrogen has three different can mass numbers. hydrogen H1 deuterium H2 C12 C14 (used in carbon dating) tritium H3