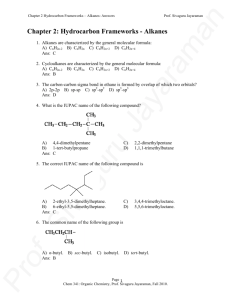
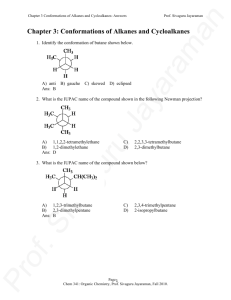
Key - The Siva Group
advertisement
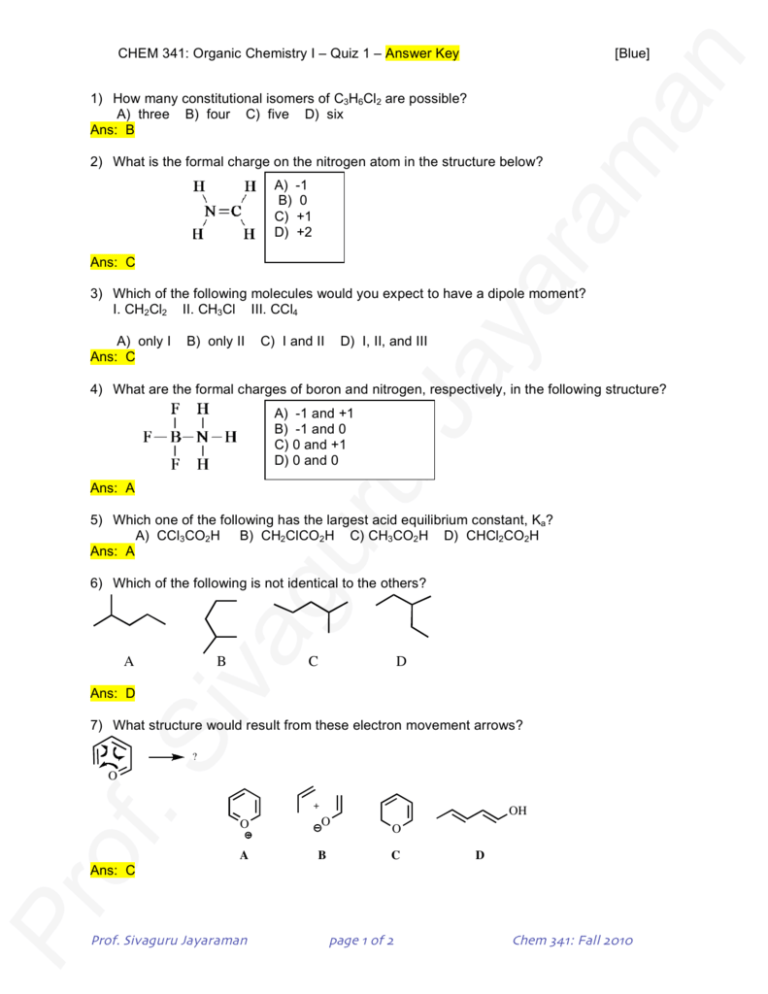
CHEM 341: Organic Chemistry I – Quiz 1 – Answer Key [Blue] 1) How many constitutional isomers of C3H6Cl2 are possible? A) three B) four C) five D) six Ans: B 2) What is the formal charge on the nitrogen atom in the structure below? A) -1 B) 0 C) +1 D) +2 Ans: C 3) Which of the following molecules would you expect to have a dipole moment? I. CH2Cl2 II. CH3Cl III. CCl4 A) only I Ans: C B) only II C) I and II D) I, II, and III 4) What are the formal charges of boron and nitrogen, respectively, in the following structure? A) B) C) D) -1 and +1 -1 and 0 0 and +1 0 and 0 Ans: A 5) Which one of the following has the largest acid equilibrium constant, Ka? A) CCl3CO2H B) CH2ClCO2H C) CH3CO2H D) CHCl2CO2H Ans: A 6) Which of the following is not identical to the others? A B C D Ans: D 7) What structure would result from these electron movement arrows? ? O + O O A B OH O C D Ans: C Prof. Sivaguru Jayaraman page 1 of 2 Chem 341: Fall 2010 CHEM 341: Organic Chemistry I – Quiz 1 – Answer Key [Blue] 8) Which of the following is not a resonance structure of O O O O (A) C O O O O (B) C O (C) O (D) Ans D 9) The molecule with the largest dipole moment is? F F F F F F (A) (B) F (C) F (D) Ans D 10) Which of the following reactions shows the correct use of "curly arrows"? + (A) O + O + Cl + Cl O Cl + (C) Cl O Cl (B) + O O Cl + (C) + O Cl O Cl Ans B 11) Bonus question Which one of the following is the strongest base? (Note: The conjugate acid of the base is the week one, so look at which one is the week conjugate acid) NH2 OH F (A) (B) Ans: A (C) (D) H3C: Prof. Sivaguru Jayaraman page 2 of 2 Chem 341: Fall 2010