The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
Contents lists available at ScienceDirect
The International Journal of Biochemistry
& Cell Biology
journal homepage: www.elsevier.com/locate/biocel
Review
Actin cytoskeleton dynamics and the cell division cycle
Yi-Wen Heng, Cheng-Gee Koh ∗
School of Biological Sciences, Nanyang Technological University, 60 Nanyang Drive, Singapore 637551, Singapore
a r t i c l e
i n f o
Article history:
Received 21 January 2010
Received in revised form 12 April 2010
Accepted 14 April 2010
Available online 20 April 2010
Keywords:
Actin cytoskeleton
Cell cycle
Mitosis
Rho GTPases
Focal adhesion
a b s t r a c t
The network of actin filaments is one of the crucial cytoskeletal structures contributing to the morphological framework of a cell and which participates in the dynamic regulation of cellular functions. In
adherent cell types, cells adhere to the substratum during interphase and spread to assume their characteristic shape supported by the actin cytoskeleton. This actin cytoskeleton is reorganized during mitosis
to form rounded cells with increased cortical rigidity. The actin cytoskeleton is re-established after mitosis, allowing cells to regain their extended shape and attachment to the substratum. The modulation of
such drastic changes in cell shape in coordination with cell cycle progression suggests a tight regulatory
interaction between cytoskeleton signalling, cell–cell/cell–matrix adhesions and mitotic events. Here,
we review the contribution of the actin cytoskeleton to cell cycle progression with an emphasis on the
effectors responsible for the regulation of the actin cytoskeleton and integration of their activities with
the cell cycle machinery.
© 2010 Elsevier Ltd. All rights reserved.
Contents
1.
2.
3.
4.
5.
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1622
Actin cytoskeleton, myosin and the cell cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1623
2.1.
Actin cytoskeleton in cell cycle control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1623
2.2.
Actin, myosin and the regulation of the mitotic spindle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1623
2.3.
Septin, actin cytoskeleton, and the cell cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1627
Rho GTPases, their regulators and the cell cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1627
3.1.
RhoA and partners in cytokinesis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1627
3.2.
RhoA and partners in other stages of mitosis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1628
3.3.
Cdc42 and partners . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1629
3.4.
Cyclin-dependent kinase and Rho GTPases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1629
Cell attachment and the cell cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1629
4.1.
Integrin signalling and the cell cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1630
4.2.
Cadherin signalling and the cell cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1630
4.3.
Focal adhesion proteins and the cell cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1630
Conclusion and perspective . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1631
Acknowledgements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1631
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1631
1. Introduction
Abbreviations: APC, adenomatous polyposis coli; CDK1, cyclin-dependent kinase
1; ECM, extra cellular matrix; FAK, focal adhesion kinase; GAP, GTPase-activating
protein; GEF, guanine nucleotide exchange factor; MLCK, myosin light chain kinase;
PAK, p21-activate kinase; Plk1, polo-like kinase 1; ROCK, p160-Rho-associated
coiled-coil-containing protein kinase.
∗ Corresponding author. Tel.: +65 63162854.
E-mail address: cgkoh@ntu.edu.sg (C.-G. Koh).
1357-2725/$ – see front matter © 2010 Elsevier Ltd. All rights reserved.
doi:10.1016/j.biocel.2010.04.007
The actin cytoskeleton of eukaryotic cells undergoes drastic
changes and remodelling during cell division. Interphase cells usually contain an extensive actin network but this network is rapidly
dismantled and rearranged when cells enter mitosis, giving mitotic
cells their characteristic round shape. At the end of mitosis, actin
rearranges at the cleavage furrows and forms part of the contractile
ring, which is central to the process of cytokinesis. Another mitotic
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
event in which the actin network plays an important role is the
separation of centrosomes, which is dependent on the cortical flow
of cortical actin and the myosin network. Disruption of the actin
and myosin II networks by inhibitory drugs such as latrunculin,
ROCK (p160-Rho-associated coiled-coil-containing protein kinase)
inhibitor and myosin II RNA interference (RNAi) causes failure in
centrosome separation and proper spindle assembly (Rosenblatt et
al., 2004; Uzbekov et al., 2002).
Thus, the regulation of the actin cytoskeleton and of cell cycle
progression appears to be connected. However, the nature of their
functional integration is not well understood. Here, we review
the current state of knowledge concerning the regulatory links
between these two activities in proliferating cells. The emphasis is
on proteins known to regulate the actin cytoskeleton and are implicated in cell cycle control. Our discussion on the actin cytoskeleton
will not be limited to the acto-myosin filament or filamentous actin
(F-actin) but will also encompass proteins associated with the modulation of the actin cytoskeleton such as the Rho GTPases and their
regulators. We will also discuss cell adhesions and their effect on
the cell cycle.
2. Actin cytoskeleton, myosin and the cell cycle
2.1. Actin cytoskeleton in cell cycle control
Actin is a highly conserved globular protein found in almost
all eukaryotic cells. It forms cellular scaffold structures that provide cells with their shape, tension support, intracellular vesicular
transport, cell attachment, adhesion properties and the ability to
move. Apart from these well-studied mechanical functions, actin
also plays a more subtle role in chemical signal transduction. It
was once thought that the cell cycle machinery controls the state
of actin organization within the cell via an “inside-out” signalling
mechanism (Wang, 1991; Yamashiro et al., 1991). However, retrograde signalling where the state of actin organization within the cell
controls cell cycle progression has proven to be important as well
(Assoian and Zhu, 1997; Thery and Bornens, 2006). The significance
of the actin cytoskeleton for cell cycle progression can be easily
gleaned from experiments using drugs or chemicals that interfere
with the actin filament in the cells. Depolymerization of actin filaments by toxins such as cytochalasin D and latrunculin B has been
reported to delay progression of mitosis in primary cells and fission
yeast, suggesting that an intact actin cytoskeleton may be required
for the efficient onset of mitosis (Gachet et al., 2001; Lee and Song,
2007). A summary of the different drugs and chemicals and their
effect on the actin cytoskeleton is listed in Table 1. While a morphogenesis checkpoint has been proposed in budding yeast which
is activated in response to perturbation of the actin cytoskeleton leading to delays in chromosome segregation (McMillan et al.,
1998), a similar actin regulated checkpoint control has not been
established in mammalian cells. Apart from causing a delay in mitosis, disruption of actin filaments also leads to G1 arrest. This actin
cytoskeleton dependent arrest has been linked to cyclin expression and cyclin-dependent kinase (CDK) activation (Reshetnikova
et al., 2000). In a study in which disruption of the actin cytoskeleton was induced by the over-expression of cofilin, a member of
the actin depolymerization factor (ADF)/cofilin family, more than
90% of H1299 lung carcinoma cells arrested at G1 phase of the cell
cycle (Lee and Keng, 2005). Excessive polymerization of F-actin by
a mutant WASP or the drug Jasplakinolide, which interferes with
actin depolymerization, causes an increase in multinucleate cells
suggesting a possible defect in cytokinesis (Moulding et al., 2007).
Similarly, expression of mutant WASPI294T which mis-regulates
the Arp2/3 complex and enhances F-actin polymerization, results
in abnormal accumulation of F-actin around the mitotic chromo-
1623
somes and may possibly lead to the observed cytokinesis defects.
These observations demonstrate actin’s involvement in cell cycle
progression. Various proteins known to function in both the regulation of the actin cytoskeleton and the cell cycle progression are
summarized in Table 2. Many of these proteins change their cellular
localization at different phases of the cell cycle (Fig. 1 and Table 3).
Recently, cortactin, an actin-binding protein, has been identified
as an anchor between the centrosome and F-actin and is essential
for F-actin driven centrosome separation during mitosis. The triply
phosphorylated form (Tyr421-, Tyr466- and Tyr482-) of cortactin
is found to be localized exclusively to the spindle poles during transition to anaphase. Truncated cortactin lacking its actin-binding
domain inhibits centrosome separation (Wang et al., 2008). Interestingly, cortactin has also been identified as a substrate of CDK1 at
serine 405 (Blethrow et al., 2008). Although the significance of this
phosphorylation of cortactin during mitosis has not been examined, it raises the possibility of a link between CDK1 signalling and
cortactin-mediated centrosomes separation during mitosis.
Links between actin cytoskeleton and transcription control have
also emerged. Disruption of the actin cytoskeleton during mitosis
leads to changes in the G- to F-actin ratio and hence in transcription
activities mediated by the myocardin-related transcription factor
and serum response factor, MAL/SRF (Miralles et al., 2003). It is
possible that cell cycle progression could be affected by the status of
actin polymerization via MAL/SRF mediated transcription. A recent
report shows that in human uterine leiomyosarcoma cells, downregulation of MAL leads to reduction of p21 CDK inhibitor (Kimura
et al., 2010). However, work by Triesman’s group has shown that
depletion of SRF or MAL affects cell spreading and adhesion without
affecting cell proliferation or apoptosis (Medjkane et al., 2009).
2.2. Actin, myosin and the regulation of the mitotic spindle
In mitosis, microtubules have hogged the limelight with their
beautiful arrays and precisely choreographed functions in organising events from the establishment of the bipolar spindle to the
capturing, alignment and accurate segregation of chromosomes.
Most importantly, mitotic spindle assembly and chromosome segregation can be reconstituted in vitro in cell free extract. The
studies on actin in mitosis have remained focused almost solely
on its mechanical function during cytokinesis until recently, where
actin’s role in the biogenesis of the mitotic spindle has gained
increasing attention. Using different methods of interference with
actin polymerization and actin-myosin at the cell cortex, two earlier papers clearly showed the requirement of myosin II and actin in
centrosome separation in higher eukaryotic and mammalian cells
(Rosenblatt et al., 2004; Uzbekov et al., 2002). Upon actin depolymerization with latrunculin treatment, centrosome separation is
blocked and a proper spindle cannot be assembled (Uzbekov et al.,
2002). Proper spindle assembly is also disrupted when myosin II is
inhibited through the use of the ROCK inhibitor – Y26732, which
prevents ROCK-mediated phosphorylation of myosin light chain
phosphatase, eventually blocking myosin activity. A similar effect
is seen in cells treated with blebbistatin which inhibits the ATPase
activity of non-muscle myosin II (Rosenblatt et al., 2004). Silencing
of non-muscle myosin II heavy or light chain using RNA interference also disrupts spindle formation. When the cortical flow of
acto-myosin filaments is prevented by cross-linking the cell surface with lectins such as concanavalin A, centrosome separation
and movement are impeded, which results in a lopsided spindle
(Rosenblatt et al., 2004).
More recent studies have illustrated a closer direct link between
the actin filaments and the mitotic spindles where F-actin was
found localized to the mitotic apparatus (Woolner et al., 2008;
Yasuda et al., 2005). Additional reports have also suggested that
the stability of the cortical actin network is crucial in establishing
1624
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
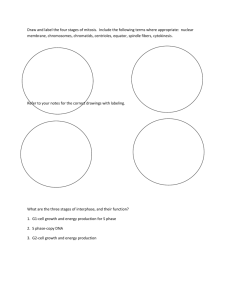
Fig. 1. Different localization of proteins at interphase and mitosis. HeLa cells were harvested at different stages of the cell cycle and immunostained with (A) Top panels:
anti-␣-tubulin antibodies. Bottom panels: phalloidin for F-actin. (B) Non-muscle myosin II heavy chain antibodies (red), DAPI (blue). (C) Top panels: anti-phospho LIMK1
(T508)/LIMK2 (T505) antibodies (green). Bottom panels: Merged image with anti-␣-tubulin (red) and DAPI stain (blue). (D) Top panels: anti-phospho PAK1 (T423) antibodies
(red). Bottom panels: merged image with anti-␣-tubulin (green) and DAPI (blue). All bars: 10 m.
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
1625
Table 1
Summary of different reagents used to perturb the actin cytoskeleton and their effects on cell cycle progression.
Drug
Target
Action
Phenotype(s) reported
Reference(s)
Clostridium difficile toxin B
RhoA, Rac1, Cdc42
G2 arrest
Ando et al. (2007)
Clostridium botulinum
exoenzyme C3 transferase
RhoA, RhoB, RhoC
ROCK I, ROCK II
Slows down p21 degradation, at
mitosis caused re-spreading of
prometaphase cells
Failure in centrosome separation
Olson et al. (1995), Yamamoto et
al. (1993)
Y-27632-Rock inhibitor
ML-7, ML-9
MLCK
G1 arrest, affect spindle organization
2,3-Butanedione monoxime
(BDM)
Blebbistatin
Myosin
Glycosylates RhoA, Rac1 and
Cdc42, thereby inactivating
them.
ADP-ribosylation on
asparagine 41 in the effector
binding domain of the GTPase
Competitive binding with ATP
to active site of ROCK I and
ROCK II
Competitive binding with ATP
to active site of MLCK
Myosin ATPase inhibitor
Deng et al. (2005), Bhadriraju and
Hansen (2004)
Forer et al. (2007), Bhadriraju and
Hansen (2004), Huang et al. (1998)
Rosenblatt et al. (2004)
Calyculin A
Protein phosphatases
CEP1347
WR-PAK18
PAK1
Cytochalasin D
Actin
Latrunculin A/B
Actin
Jasplakinolide
Actin
Myosin II
Binds to Myosin-ADP-Pi with
high affinity and interferes
with phosphate release process
Binds to active site of PP1 and
PP2A protein phosphatases
Targets PAK1 ATP-binding site
Binds SH3 domain of PIX,
blocking PAK-PIX interaction
Binds to the barbed, fast
growing plus ends of
microfilaments, inhibiting
actin monomer assembly and
disassembly
Binds to actin monomers near
their ATP-binding site,
preventing actin
polymerization
Binds to F-actin, stabilizing and
promoting actin
polymerization
correct spindle orientation in mammalian cells. The activity of LIM
Kinase-1 (LIMK1) was found to increase during mitosis resulting in
the phosphorylation and inactivation of cofilin (Kaji et al., 2008).
Knockdown of LIMK1 leads to more activation and mislocalization
of cofilin, which in turn results in mis-orientation of the spindle.
Similar defects can be induced by inhibition of actin polymerization
using latrunculin A or by over-expression of a non-phophorylatable
cofilin (S3A). In addition, phosphorylated LIM kinases, but not the
unphosphorylated form, have been reported to colocalize and coimmunoprecipitate with ␥-tubulin during early stages of mitosis
(Chakrabarti et al., 2007). During metaphase, LIMK1 localises to
the centrosomes while LIMK2 associates mainly with the mitotic
spindle (Sumi et al., 2006). These data suggest that the activity of
LIM kinases may play an important role in the regulation of spindle
activity during mitosis.
Apart from the interactions between the astral microtubule
and the cortical actin network, other sites of interaction between
the spindle microtubules and acto-myosin filaments have long
been reported (Maupin and Pollard, 1986; Wu et al., 1998). More
recently, a myosin isoform Myo10, which binds to both actin and
microtubules, has been found to localize to the poles of mitotic spindles in Xenopus embryos (Woolner et al., 2008). Knocking down
Myo10 causes mitotic spindle defects which include fragmentation of the spindle poles and lengthening of the spindles. Dynamic
F-actin cables are also found localized to the mitotic spindles and
the spindle poles. The authors proposed that F-actin and Myo10
regulate spindle lengthening and shortening, respectively. While
the F-actin-mediated spindle lengthening is independent of Myo10,
spindle shortening induced by Myo10 requires F-actin. Thus F-actin
and Myo10 have both overlapping and distinct roles in mitosis.
These observations suggest that the actin and microtubule structures may act synergistically in the assembly and positioning of the
mitotic apparatus.
G1 arrest, inhibits
kinetochore-microtubule elongation
Failure in centrosome separation
Rosenblatt et al. (2004)
Accelerate anaphase chromosome
separation
G1 arrest, aberrant spindle
formation, delay in mitosis
transition
Mitosis delay, G1 arrest, inhibits
kinetochore-microtubule elongation
Fabian et al. (2007)
Mitosis delay, G1 arrest, inhibits
kinetochore-microtubule elongation,
failure in centrosome separation
Rosenblatt et al. (2004), Uzbekov
et al. (2002), Gachet et al. (2001),
Lee and Song (2007)
Cytokinesis defect
Moulding et al. (2007)
Nheu
et
al.
(2004)
Gachet et al. (2001), Lee and Song
(2007), Forer et al. (2007)
Besides spindle formation, actin and myosin can exert their
effects at different stages of the cell division. For example,
myosin light chain kinase (MLCK) has been shown to function
in the phosphorylation of myosin II which is required for its
bundling with actin for contractile ring formation and for actomyosin contractility forces that are necessary for cytokinesis
(Mabuchi, 1986). MLCK activity also plays an important role in
early mitotic events. Microinjection of the catalytic fragment of
MLCK into prophase cells delays the transition from nuclear envelope breakdown to the onset of anaphase, but does not affect the
duration between anaphase onset and mid cytokinesis. Unregulated MLCK activity is also found to cause reduced fluorescent
staining of spindle microtubules in prometaphase and metaphase
cells (Fishkind et al., 1991). Similarly, treatment of mouse eggs
with MLCK inhibitor ML-7, or the auto-inhibitory Peptide 18,
affects localization of actin cap on the metaphase II spindles
and subsequent cortical reorganization activities (Deng et al.,
2005).
Although ROCK and myosin activities are required for the
rounding up of cells during mitosis (Maddox and Burridge, 2003),
the actual mechanism remains elusive. Additional evidence that
the actin structures can affect cell morphology during mitosis
came from the study of moesin. Moesin is a member of the
ezrin/radixin/moesin (ERM) family of actin-binding proteins and
has been implicated in cell rounding in mitosis. Phosphorylation of
moesin by the Ste20-like protein kinase Slik in Drosophila S2 cells
causes cell rounding in mitosis mainly via cross-linking actin to
the membrane at the cortex to increase cortical rigidity (Carreno
et al., 2008; Kunda et al., 2008). Knocking down of moesin and
its upstream kinase Slik leads to defects in cell cortex organization as well as metaphase spindle stability. Increasing cell rigidity
externally by using lectin to crosslink the cell membrane can partially rescue the moesin knockdown phenotype suggesting that
1626
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
Table 2
Summary of the role of different groups of proteins in actin cytoskeleton regulation and cell cycle progression.
Protein Group
Protein
Roles in actin cytoskeleton
regulation
Roles in cell cycle progression
regulation
Reference(s)
Myosin II
Actin crosslinker; ATPase
dependent acto-myosin force
generation.
Rosenblatt et al. (2004),
Uzbekov et al. (2002), Mabuchi
(1986), Fabian et al. (2007)
Myosin X
Anillin
Unconventional actin crosslinker.
Actin and microtubule bundling.
Cortactin
Recruitment of Arp2/3 to F-actin.
Moesin
Actin binding; ERM protein.
Septin
RhoA
Actin binding; scaffold for
non-muscle MyoII and its
kinases.
Stress fibres formation.
G1 -S progression; mitotic cell
rounding; centrosome separation;
mitotic spindle assembly;
kinetochore-microtubule formation;
anaphase chromosome movement;
cytokinesis.
Mitotic spindle shortening.
Spindle associated cleavage
specification.
F-actin mediated centrosome
separation.
Mitotic cell rounding; mitotic cortical
rigidity; metaphase spindle stability.
Cytokinesis; chromosome congression
and segregation at mitosis
Rac1
Lamellapodia formation.
G1 -S transition; cytokinesis.
Cdc42
Filopodia formation.
p190RhoGAP
Ect2
RhoA GAP.
RhoA GEF, Cdc42 GEF during
mitosis.
G1 -S transition;
kinetochore-microtubule stabilization;
spindle biorientation; metaphase
chromosome alignment; cytokinesis.
Mitotic cell rounding.
Contractile ring formation; contraction
of contractile ring at cleavage furrow.
GEF-H1
RhoA GEF; microtubule
dynamics.
Rac1 and Cdc42 GAP; RhoA GAP
during mitosis.
Actin binding
Rho GTPase
Rho GTPase Regulator
MgcRacGAP
Rho GTPase effector and
down-stream target
Lfc
MyoGEF
RhoA GEF.
RhoA GEF; myosin II binding.
ROCK
RhoA effector; myosin
regulation; actin bundling.
LIMK1
LIMK2
Cofilin
ROCK & PAK substrates; cofilin
regulation; actin bundling.
Downstream of RhoA pathway;
actin severing.
RhoA effector; actin nucleation
and elongation.
mDia1
PRK2/PKN2
PAK1
RhoA and Rac effector.
Rac and Cdc42 effector; cell
motility; focal adhesion turnover.
N-WASP
Rac and Cdc42 effector; Arp2/3
activator; actin nucleation.
the maintenance of cell shape and rigidity is sufficient to stabilize the mitotic spindles. Moesin’s function in rounding up of cells
appears to be independent of myosin II because cells expressing
active T559D moesin but lacking myosin light chain are still able
to round up during mitosis (Kunda et al., 2008). However, it is
very likely that both moesin-actin and myosin-actin activities are
required to establish the rounded cell shape and rigidity for proper
spindle assembly and positioning.
Actin and myosin structures also participate in generating
the forces required for chromosome segregation. Treatment of
crane-fly spermatocytes with actin depolymerization drugs such
as cytochalasin D and lantrunculin A or myosin ATPase inhibitors
like butanedione monoxime (BDM) causes inhibition of spindle
microtubule elongation (Forer et al., 2007). Conversely, nonspecific inhibition of myosin light chain phosphatase by calyculin A
G1 -S transition; mitotic cell rounding;
mitotic cortical rigidity; cytokinesis.
RhoA related cytokinesis.
Formation of ingression furrow;
interacts with Ect2.
Mitotic spindle assembly.
Localize RhoA and Ect2 to contractile
ring.
S phase progression, mitotic cell
rounding; centrosome separation;
mitotic spindle assembly; cytokinesis.
Mitotic spindle orientation.
Regulate cyclin A and p27 expression.
G1 -S progression; mitotic spindle
orientation.
Spindle formation in early mitosis
through an “Lfc-RhoA-mDia1”
pathway.
Mitosis entry; mitosis exit.
G1 -S transition; G2 -M transition;
centrosome maturation; regulation of
Plk1 and Aurora-A activity; regulate
astral microtubule dynamics; spindle
orientation.
Cytokinesis.
Woolner et al. (2008)
Piekny and Glotzer (2008),
Gregory et al. (2008)
Wang et al. (2008)
Carreno et al. (2008), Kunda et
al. (2008)
Joo et al. (2007), Spiliotis et al.
(2005)
Coleman et al. (2006),
Nishimura and Yonemura
(2006), Birkenfeld et al. (2007)
Canman et al. (2008), Klein et
al. (2009)
Yasuda et al. (2004, 2006), Jaffe
et al. (2008), Mitsushima et al.
(2009)
Maddox and Burridge (2003)
Nishimura and Yonemura
(2006), Oceguera-Yanez et al.
(2005)
Birkenfeld et al. (2007)
Minoshima et al. (2003), Zhao
and Fang (2005), Miller and
Bement (2009)
Bakal et al. (2005)
Asiedu et al. (2009)
Rosenblatt et al. (2004),
Uzbekov et al. (2002), Croft and
Olson (2006)
Kaji et al. (2008)
Croft and Olson (2006)
Lee and Keng (2005), Kaji et al.
(2008)
Bakal et al. (2005)
Schmidt et al. (2007)
Maroto et al. (2008), Zhao et al.
(2005), Vadlamudi et al.
(2000), Nheu et al. (2004),
Balasenthil et al. (2004)
Moulding et al. (2007)
results in the stabilization of myosin II phosphorylation and the
subsequent acceleration of pole-ward movement of chromosomes
during anaphase (Fabian et al., 2007).
These studies strongly imply that the formation of mitotic
spindle is highly dependent on the actin and myosin networks
within the cell. Actin reorganization during mitosis helps create the cellular environment required for the mitotic spindle
to serve its function. Disruption of the cortical actin architecture during mitosis can severely affect spindle orientation which
may result in cell cycle arrest. There may well exist in mammalian cells, like in the budding yeast, a morphogenesis checkpoint
which is dependent on the actin cytoskeleton (Lew, 2003) or
a spindle orientation checkpoint (Gachet et al., 2006) which
hinges on the interaction between the microtubule and cortical
actin, although the existence of a spindle orientation checkpoint
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
1627
Table 3
The localization of different proteins linked to the actin cytoskeleton in interphase and mitosis.
Protein
F-actin
Myosin II
Anillin
Septin
Cortactin
Ect2
GEF-H1
LIMK1
LIMK2
MyoGEF
PAK1
RhoA
HEF1
Integrin-linked kinase (ILK)
Focal adhesion kinase (FAK)
Pyk2
Paxillin
Zyxin
Subcellular localization by immunofluorescence staining
Interphase (adherent cell type)
M-phase
Stress fibers; actin structures
Stress fibers; actin structures
Ubiquitous
Stress fibres
Ubiquitous, actin structures
Ubiquitous
Ubiquitous
Cell–cell contacts
Ubiquitous
Ubiquitous
Ubiquitous; actin structures; centrosomes
Ubiquitous
Focal adhesions
Focal adhesions
Focal adhesions
Focal adhesions
Focal adhesions
Focal adhesions
Contractile ring
Contractile ring
Contractile ring
Contractile ring
Centrosomes (phosphorylated from)
Central spindle
Mitotic spindle; midzone
Centrosomes; equatorial cortex; contractile ring
Mitotic spindle, contractile ring
Central spindle
Centrosomes; contractile ring; mid-body
Contractile ring
Mitotic spindle; mid-body
Centrosomes
Centrosones
Centrosomes
Centrosomes
Mitotic spindle; central spindle
in the fission yeast is now questionable (Meadows and Millar,
2008).
2.3. Septin, actin cytoskeleton, and the cell cycle
Septins are a family of GTPase which can polymerize to form filamentous structures. They were originally discovered in the screen
for cell division mutants in the budding yeast (Hartwell, 1971).
Their main role is in the control of cytokinesis. A ring of septin
polymers is assembled during early stages of the cell cycle at the
bud neck and remains till cytokinesis (Cid et al., 2001). Apart from
cytokinesis, septins have also been implicated in the regulation of
GIN4 kinase activation required for bud growth (Carroll et al., 1998).
Mammalian septins are found to associate with the plasma
membrane, actin cytosketon and the microtubules (Spiliotis and
Nelson, 2006). Mammalian Septin2 colocalizes with actin stress
fibres during the interphase and the contractile ring at cytokinesis. It has also been shown to bind to non-muscle myosin II (Joo
et al., 2007). In HeLa and MDCK cells, septins are localized to the
metaphase plate during mitosis (Spiliotis et al., 2005). Knocking
down of septins results in loss of chromosomes from the metaphase
plate. It has been suggested that the septins form a scaffold at the
midplane of mitotic spindle to maintain CENP-E motor protein at
the kinetochores thereby facilitating the congression of chromosomes at the metaphase plate. Interestingly, Kremer et al. have
shown that septins present in the cytoplasm bind to and act as a
reservoir for SOCS7 (suppressor of cytokine signalling 7). Knocking down of septins 2, 6 and 7 causes loss of stress fibres and
also nuclear accumulation of NCK (Kremer et al., 2007). SOCS7 is
responsible for the import of NCK into the nucleus. DNA damages also result in the nuclear accumulation of SOCS7 and NCK.
Hence septins are also linked to the DNA damage checkpoint via
the septin-SOCS7-NCK pathway. Given that NCK is associated with
the control of actin cytoskeleton, there is a possibility that cell cycle
progression and actin cytoskeleton can influence each other via this
pathway.
3. Rho GTPases, their regulators and the cell cycle
The small GTPases belonging to the Rho family have long
been associated with the regulation and remodelling of the actin
cytoskeleton and are important for cell motility, morphogenesis and neurite development (Etienne-Manneville and Hall, 2002;
Koh, 2006). Active RhoA causes the formation of stress fibres by
Reference(s)
Schroeder (1968)
Mabuchi and Okuno (1977)
Piekny and Glotzer (2008)
Joo et al. (2007)
Wang et al. (2008)
Nishimura and Yonemura (2006)
Birkenfeld et al. (2007)
Sumi et al. (2006)
Sumi et al. (2006)
Asiedu et al. (2009)
Zhao et al. (2005)
Yuce et al. (2005)
Law et al. (1998)
Fielding et al. (2008)
Rodriguez-Fernandez et al. (1999)
Rodriguez-Fernandez et al. (1999)
Herreros et al. (2000)
Hirota et al. (2000)
increasing acto-myosin contraction and bundling of actin filament
whereas active Cdc42 and Rac1 increase filopodia and lamellipodia formation, respectively. Apart from their roles in modulating
the actin cytoskeleton, Rho GTPases have also been found to regulate cell cycle progression with two main sites of action: one at
the G1 /S transition and the other during cytokinesis. For example,
inhibition of Cdc42, Rac1 and RhoA results in cell cycle arrest at G1
phase of the cell cycle (Olson et al., 1995; Yamamoto et al., 1993). In
human capillary endothelial cells, active RhoA causes an increase
in the expression of the F-box containing protein Skp2 which is
required for ubiquitinylation-dependent degradation of the CDK
inhibitor p27kip1 (Mammoto et al., 2004). p27kip1 binds to and inactivates the cyclin D1/CDK4 and cyclin E/CDK2 complexes. Absence
of active RhoA leads to high levels of p27kip1 , resulting in cell cycle
arrest in G1 . Active RhoA acts via the balance of activities of its
two effectors, ROCK and mDia, to activate the Skp2-p27kip1 pathway. It has also been shown that inhibition of RhoA or disruption
of F-actin drastically slows down the degradation of another CDK
inhibitor p21Waf/Cip1 (Coleman et al., 2006). Specifically, ROCK has
been shown to regulate cyclin A expression via the Ras/MAPK pathway and via LIMK2 (Croft and Olson, 2006). More recently, a study
using the Clostridium difficile toxin B to inhibit the Rho GTPases at
the G2 phase of the cell cycle also reveals possible involvement
of the Rho GTPases in the control of multiple signalling pathways
involved in the progression to mitosis (Ando et al., 2007). The different pathways regulated by Rho GTPases at different phases of
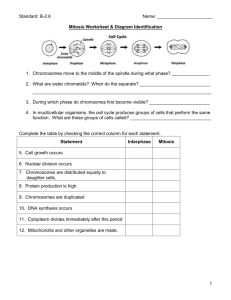
the cell cycle are summarized in Fig. 2.
3.1. RhoA and partners in cytokinesis
Although RhoA activities have been reported to be required for
cell cycle progression at different phases, most studies have concentrated on the role of RhoA in cytokinesis where the activity
of RhoA is essential. RhoA organises the assembly of the contractile ring and induces the acto-myosin-driven constriction of the
cleavage furrow [reviewed by Barr and Gruneberg, 2007]. ECT2,
a guanine nucleotide exchange factor (GEF) which activates RhoA,
localizes and activates RhoA at the cleavage furrow (Nishimura and
Yonemura, 2006). GEF-H1, a microtubule-regulated GEF for RhoA,
has also been shown to modulate RhoA activity during cytokinesis (Birkenfeld et al., 2007). These GEFs are, in turn, activated by
CDK1/cyclin B and Aurora-A/B kinases (Birkenfeld et al., 2007; Hara
et al., 2006; Niiya et al., 2006). Through the use of chemical genetics
and specific inhibitors, Polo-like kinase Plk1, originally thought to
1628
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
Fig. 2. Functional connections between proteins involved in the regulation of Rho GTPases, the actin cytoskeleton and cell cycle progression. The activities of different
proteins and how they can influence one another at different phases of the cell cycle are summarized.
regulate only spindle assembly, has been found to also control the
localization of ECT2 to the central spindle and RhoA at the equator
in anaphase (Brennan et al., 2007; Burkard et al., 2007; Petronczki et
al., 2007). Inhibition of Plk1 blocks the interaction of ECT2 with the
midzone anchor HsCyk4 and the assembly of the contractile ring,
resulting in the inhibition of cytokinesis and the formation of binucleate cells. Recently, centrosome/spindle pole-associated protein
(CSPP) has been found to target MyoGEF (Myosin II-interacting GEF)
to the central spindle during anaphase. MyoGEF also interacts with
ECT2. Knockdown of MyoGEF results in mislocalization of ECT2
and RhoA during cytokinesis (Asiedu et al., 2009). Centralspindlin
which is localized to both the central spindle microtubules and the
tips of astral microtubules near the equatorial cortex is reported
to recruit ECT2 to the central spindle (Nishimura and Yonemura,
2006). Knockdown of centralspindlin component, MKLP1, causes
failure of ECT2 to localize to the equatorial cell cortex (Yuce et al.,
2005).
Another Rho GTPase regulator which participates in the control of cytokinesis and contractile ring assembly is MgcRacGAP
(also known as HsCyk4). MgcRacGAP is a GTPase-activating protein for Rac and Cdc42 but is converted to a GAP for RhoA when
phophorylated by Aurora-B in M phase of the cell cycle (Minoshima
et al., 2003). Silencing of MgcRacGAP by RNAi results in the loss of
ingression of the cleavage furrow and hence failure of the cells to
undergo cytokinesis (Zhao and Fang, 2005). MgcRacGAP interacts
with ECT2 and therefore may exert its effect via ECT2. A more recent
report proposes a model where the GAP activities of MgcRacGAP
and GEF activity of ECT2 act concurrently to promote a flux of RhoA
activities, thereby maintaining a RhoA-rich zone at the cell equator
(Miller and Bement, 2009).
Anillin, a 124 kDa RhoA binding protein, has been shown to accumulate at the cleavage furrow in a RhoA dependent manner (Piekny
and Glotzer, 2008). Anillin was first isolated as an actin binding and
bundling protein in Drosophila embryo (Field and Alberts, 1995).
It was later shown to interact with myosin regulatory light chain
(MLC) and septin (Kinoshita et al., 2002; Oegema et al., 2000;
Straight et al., 2005). A recent report reveals that in Drosophila the
RhoGEF pebble controls the formation of a filamentous structure
containing Rho1, anillin and septin at the cleavage furrow, as well
as the interaction of this filamentous structure with the plasma
membrane and microtubules (Hickson and O’Farrell, 2008). Interestingly, anillin also interacts with RacGAP50C, which is a spindle
associated protein that specifies the cleavage site (Gregory et al.,
2008). Depletion of anillin results in the loss of RacGAP50C from
the equator of cleavage and the collapse of the cleavage furrow.
Thus anillin acts as a linker between the contractile ring and the
spindle.
3.2. RhoA and partners in other stages of mitosis
Other than cytokinesis, RhoA activities are also required in other
phases of the cell cycle. Microtubule-associated Rho GEF Lfc has
been implicated in spindle formation in early mitosis in Rat2 fibroblast cells (Bakal et al., 2005). Lfc knockdown or microinjection
of anti-Lfc antibody causes spindle assembly defects in the early
stages of mitosis. Both active RhoA and the formin mDia1 can res-
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
cue these defects, suggesting that an “Lfc-RhoA-mDia1” pathway
is working in Rat2 cells for spindle formation during early mitosis. Additionally, via the use of novel covalent capturing method of
kinase-specific phosphopeptides, mDia1 was found to contain an
optimal CDK1 phosphorylation consensus sequence (Blethrow et
al., 2008), suggesting a possible direct connection between CDK1
and mDia1 during mitosis.
In addition to mDia1 and ROCK, another RhoA effector,
PRK2/PKN2, has also been implicated in the onset of mitosis and the
completion of cytokinesis (Schmidt et al., 2007). Silencing of PRK2
in HeLa S3 cells leads to accumulation of bi-nucleated cells and
these cells also show delay in G2 /M progression. PRK2 contributes
positively towards the phosphorylation of Cdc25B, leading to the
activation of CDK1/cyclin B. These effectors appear to constitute
a feedback loop as active CDK1/cyclinB can further phosphorylate
PRK2 which can then participate in the abscission process during
cytokinesis.
3.3. Cdc42 and partners
The level of the guanosine 5 -triphosphate (GTP) bound form
of Cdc42 changes during cell cycle progression. The abundance of
Cdc42-GTP is low during pre-metaphase, peaks during metaphase
and declines again at telophase. However, there is no change in
Rac-GTP levels throughout the cell cycle (Oceguera-Yanez et al.,
2005). The level of RhoA-GTP, on the other hand, peaks at telophase.
Over-expression of dominant-negative ECT2 and MgcRacGAP as
well as silencing of ECT2 by RNAi prevented the change in the
level of Cdc42-GTP in mitosis (Oceguera-Yanez et al., 2005). These
observations suggest that both ECT2 and MgcRacGAP can regulate
both RhoA and Cdc42 at different stages of the cell cycle. More
specifically, while ECT2 catalyses the formation of Cdc42-GTP at
metaphase, MgcRacGAP increases the hydrolysis of Cdc42-GTP at
prometaphase.
The spatial and temporal function of Cdc42-GTP is required for
the progression through mitosis. Reduction in the activity of Cdc42
by RNAi causes a delay in mitotic progression. When Cdc42 RNAi
is combined with the knockdown of other Cdc42-like GTPases, a
high proportion of the cells showed misalignment of chromosomes
(Yasuda et al., 2006). It has been suggested that one of the major
roles of Cdc42 in mitosis is to control biorientation and stabilization
of the kinetochore-microtubule attachment via its association and
activation of the formin protein mDia3 (Yasuda et al., 2004), rather
than the modulation of the actin cytoskeleton. Another formin protein mDia2 is reported to have microtubule stabilizing activities,
which is independent of its actin nucleation activity (Bartolini et
al., 2008). mDia2 has been shown to bind to microtubules directly
as well as to the microtubule tip proteins such as EB1 and APC. The
actin cytoskeleton related role for mDia2 in the regulation of cell
cycle has been attributed to the stabilization of the actin scaffold
for the contractile ring during cytokinesis (Watanabe et al., 2008).
The p21-activated kinases (PAKs), a family of serine-threonine
kinases which are effectors of Cdc42 and Rac1, have been implicated in the control of G2 /M transition. It has been shown that
PAK1 regulates Plk1 activity. Inhibition of PAK1 activity leads to
a delay in G2 /M and aberrant spindle assembly which are also the
phenotypes that result from Plk1 inactivation (Maroto et al., 2008).
PAK1 has also been shown to activate Aurora-A kinase at the centrosome (Zhao et al., 2005). PAK1 is targeted to the centrosome by
the PIX-GIT1 complex. Studies have also proposed that PAK1 localization to the centrosome during metaphase to anaphase transition
requires its kinase activity as the expression of a kinase inhibitory
domain, PAK1-KID, causes a drastic reduction in centrosomal targeting (Li et al., 2002). When PAK1 is activated at the centrosome,
it dissociates from PIX-GIT1 and is able to phosphorylate and activate Aurora-A. Aurora-A activation is required for the maturation
1629
of the centrosome in the late G2 phase. PAK1 activity may also play
an important role in the regulation of astral microtubule dynamics during mitosis since over-expression of active PAK1 resulted in
multiple spindle orientations (Vadlamudi et al., 2000).
Studies have also linked PAK1 signalling to G1 to S phase transition via the regulation of the cyclin D1 machinery. It has been shown
that in Ras transformed NIH 3T3 cells, the addition of two distinct
and specific PAK1-3 inhibitors, CEP-1347 and WR-PAK18 was able
to block malignant growth by down-regulation of cyclin D1 (Nheu
et al., 2004). Consistent with this, perturbation of PAK1 activity by
PAK1-KID, or knockdown of PAK1 by siRNA resulted in a marked
decrease in cyclin D1 expression (Balasenthil et al., 2004). So far,
a direct linkage of Cdc42 or Rac1 activity to these activities of PAK
has not been documented, even though a concomitant increase in
Cdc42 and PAK1 activity has been reported (Oceguera-Yanez et al.,
2005). Thus PAK1 may exhibit its function in a GTPase-dependent
or independent pathway.
Two recent papers have illustrated that Cdc42 is important in
controlling spindle orientation in mitotic cells (Jaffe et al., 2008;
Mitsushima et al., 2009). Deletion of Cdc42 did not affect cell polarity but instead caused mis-orientation of the spindle leading to
inappropriate positioning of the apical surfaces after cell division
(Jaffe et al., 2008). Mitsushima et al. (2009) went further to demonstrate that two independent pathways downstream of Cdc42 are
involved in regulating spindle orientation: one involving Cdc42PAK2-PIX and the other involving phosphatidylinositol 3 kinase
(PI3K). It appears that the two independent pathways collectively
affect the phosphatidyl 3,4,5 triphosphate (PIP3) levels and the
cortical actin structures (Mitsushima et al., 2009).
Not much has been reported about Rac1 and its role in the control of the cell cycle. One recent report suggests that the inhibition
of Rac by the CYK-4/MgcRacGAP of the centralspindlin complex is
essential for cytokinesis in C. elegans (Canman et al., 2008). Depletion of Rac but not RhoA can rescue the cytokinesis defect of a CYK-4
GAP mutant. This work suggests a parallel inhibition of Rac and activation of RhoA during cytokinesis, most probably to prevent the
activation of Arp2/3 complex by WASP or WAVE which is downstream of Rac. The net result is the prevention of the formation of
other actin networks which may interfere with the contractile ring.
3.4. Cyclin-dependent kinase and Rho GTPases
That the onset of mitosis requires activation of CDK1 and is
accompanied by drastic rearrangement of the actin cytoskeleton
leading to the rounding up of the cell, suggests an intimate regulatory connection between CDK1 and actin cytoskeleton. While
the exact signalling pathways of CDK1 activity leading to mitotic
cytoskeletal changes remain poorly understood, p190 Rho GTPaseactivating protein (GAP) has been proposed as a major downstream
effector of CDK1 (Maddox and Burridge, 2003). Activated CDK1
phosphorylates p190RhoGAP, down regulating its activity and thus
decreases GTP hydrolysis by RhoA. This triggers a signalling cascade
through ROCK and MLC phosphatase, that regulates cytoskeleton
rearrangement observed in mitosis (Amano et al., 1996; Maddox
and Burridge, 2003).
4. Cell attachment and the cell cycle
Attachment of cells to the extracellular matrix (ECM) or other
cells has long been implicated in cell cycle regulation. During cell
division, the cells undergo extensive cell shape changes to detach
from and reattach to the ECM. While cell–matrix adhesions have
been reported to reduce during mitosis, cell–cell adhesions via the
desmosomes, tight junctions and zonulae adherentes in epithelial
cells are maintained throughout cell divisions (Baker and Garrod,
1630
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
1993; Jinguji and Ishikawa, 1992; Reinsch and Karsenti, 1994). In
mammalian cells, inter-dependence of cell attachment and cell
cycle signalling can be attributed to integrin and cadherin signals
(Pugacheva et al., 2006). Knocking-out or silencing of focal adhesion proteins vinculin, paxillin and the adaptor protein CRK has
been shown to result in the fusion of daughter cells leading to binucleate cells, demonstrating that these proteins are essential for
the completion of cytokinesis (Nagasaki et al., 2009; Shafikhani et
al., 2008).
A recent paper reported a positive correlation between ECM
stiffness and progression into S phase of the cell cycle in mammary epithelial cells and vascular smooth muscle cells (Klein et
al., 2009). Using hydrogel to simulate physiological stiffness, it has
been shown that an increase in matrix stiffness results in selective
integrin activation, leading to localization and activation of focal
adhesion kinase (FAK) which eventually induces RAC1 activation
and cyclin D1 expression.
4.1. Integrin signalling and the cell cycle
Integrins associate with numerous proteins and localise at the
focal contacts. Upon engagement with ECM, they activate proximal signalling proteins such as FAK, SRC and CAS family members,
which in turn signal through several signalling cascades, e.g. the
RAP and B-RAF pathway, the PI3K, RAC, AKT and PAK pathway,
and the SHC, GRB2, RAS and RAF pathway. These three pathways
converge to activate MEK and ERK kinases and together activate
G1 -specific cyclins D and E (Pugacheva et al., 2006). In addition,
integrin-activated FAK can directly phosphorylate transcription
factor KLF8, leading to its nuclear translocation and the activation of
cyclin D1 promoter (Zhao et al., 2003). Integrins also activate different GEFs, which lead to the activation of the Rho GTPases. However,
it has been reported that focal adhesion signalling and cell spreading are dispensable for progression through the cell cycle as long
as there is sufficient cyclin D1 (Margadant et al., 2007).
Integrin mediated cell adhesion and the ECM can also control
the orientation of the spindles and hence determine the spindle
axis and the plane of cell division (Thery et al., 2005; Toyoshima and
Nishida, 2007). 1-Integrin knockout results in random spindle orientation and a high incidence of binucleate cells (Aszodi et al., 2003;
Lechler and Fuchs, 2005). The use of an inactive 1-integrin mutant
reveals a role for integrin in bipolar spindle assembly and cytokinesis (Reverte et al., 2006). In addition to its role in focal adhesion
regulation, integrin-linked kinase (ILK) has also been localized to
the centrosome and is implicated in mitotic spindle assembly and
chromosome segregation (Fielding et al., 2008). However, it is not
clear if the roles of ILK at the focal adhesions and the centrosomes
are dependent on each other.
Integrin signalling may also serve as an important link between
the small GTPase Rap1 and mitosis. The activity of Rap1 is regulated
during mitosis. Inhibition of Rap1 is required for focal adhesion disassembly at the onset of mitosis whereas Rap1 activation is needed
for cell spreading after mitosis (Dao et al., 2009). Rap1 has also
been shown to activate many integrins (Bos, 2005; Caron, 2003). It
is very likely that Rap1 modulates integrin signalling and thereby
influences the cell shape changes which accompany mitosis.
4.2. Cadherin signalling and the cell cycle
Cadherins are Ca2+ -dependent transmembrane proteins that
participate in cell–cell adhesion. In stable adherens junctions, cadherin assembles with ␣-catenin, -catenin and actin filaments
to form stable quaternary complexes that limit cell growth via
contact-inhibition. In the absence of cell–cell contacts, -catenin
is displaced from cadherin and translocates to the nucleus thereby
activating cyclin D1 transcription (Yamada et al., 2005). Interest-
ingly, -catenin has also been shown to have an unexpected role
as a component of the inter-centrosomal linker and is essential
for the establishment of bipolar spindle (Bahmanyar et al., 2008).
Although -catenin is thought to be the major effector of cadherin engagement for cell cycle regulation, ␣-catenin has also been
reported to influence cell cycle regulation. ␣-catenin has been
shown to bind to various actin-binding proteins such as ␣-actinin
(Knudsen et al., 1995), vinculin (Hazan et al., 1997; Watabe-Uchida
et al., 1998; Weiss et al., 1998), Ajuba (Marie et al., 2003), spectrin
(Pradhan et al., 2001), ZO-1 (Itoh et al., 1997), formin (Kobielak
et al., 2004) and afadin (Pokutta et al., 2002), suggesting these
actin-binding proteins may play associative roles in the modulation of adherens junctions-mediated cell cycle control. Another
component of the adherens junctions, p120 catenin, is also implicated in the regulation of mitosis. Loss of p120 catenin results in
mitotic defects leading to extended M phase and binucleate cells
(Perez-Moreno et al., 2008). This has been attributed to the abnormally high RhoA activity in the p120 catenin conditional-knockout
cells.
Additional evidence that cell–cell adhesion plays an important
role in determining spindle orientation comes from the work of
den Elzen et al. (2009). They have reported that E-Cadherin can
provide cues to orient the mitotic spindle during symmetric cell
divisions in mammalian epithelia (den Elzen et al., 2009). When
dominant-negative E-Cadherin is introduced into MDCK cells, more
than half of the cells showed mis-oriented spindles. Moreover,
cortical APC (adenomatous polyposis coli) staining, which is consistently localized to the cell cortex at the apicolateral region, is lost in
cells expressing dominant-negative E-Cadherin. Silencing of APC by
siRNA abolishes junctional staining of APC and causes spindle misorientation, suggesting that APC may mediate cadherin signalling
to orient the mitotic spindle.
In another study using Drosophila neuroepithelial cells, symmetric mode of cell division can be converted into asymmetric
division upon the disruption of the adherens junctions. The APC
protein which is localized at the adherens junctions is involved in
the maintenance of the symmetric mode of division. The APC and
the microtubule-associated EB1 proteins function together to orient the mitotic spindle to provide the polarity cues for symmetric
division (Lu et al., 2001).
4.3. Focal adhesion proteins and the cell cycle
In adherent cell types, cell rounding upon entry into mitosis is
accompanied by a reduction in the focal contacts and an increase
in cortical rigidity. After cytokinesis, cells reattach to their substratum and re-establish cytoskeletal networks. The concerted changes
in cell shape and adhesion as cells prepare to enter mitosis suggest intense cross-signalling events between focal contacts and cell
cycle signalling. This is evident from the observation that focal contacts are more densely distributed in the proximity of signalling
molecules such as protein kinase C (Liao and Jaken, 1993), tyrosine
kinases (Hanks et al., 1992; Schaller et al., 1992) and tyrosine phosphatases (Serra-Pages et al., 1995; Shen et al., 1998). It is possible
that post translational modifications play a major role in eliciting such drastic changes within a few minutes. Indeed, HEF1, FAK,
actopaxin, paxillin and PAK are phosphorylated at mitosis-specific
sites (Clarke et al., 2004; Law et al., 1998; Yamakita et al., 1999)
during early M phase. In addition, proteins associated with focal
adhesion contacts such as FAK (Rodriguez-Fernandez et al., 1999),
Pyk2 (Rodriguez-Fernandez et al., 1999), paxillin (Herreros et al.,
2000) and zyxin (Hirota et al., 2000) are also found to associate
with the mitotic spindle or the microtubules-organizing centre during mitosis (Table 3). The dual roles focal adhesion proteins play in
adhesion-mediated signalling and mitosis-associated events suggest a precise temporal control of molecular events ensuring that
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
one set of events is completed before the next phase of the cell cycle
is initiated.
FAK serves as an important signalling protein at focal adhesion sites to mediate focal adhesion formation, cell migration and
cell cycle progression. Over-expression of FAK has been associated
with invasiveness in a variety of human tumours (Owens et al.,
1995). In interphase, FAK mediates cyclin D1 and p21 CDK inhibitor
expression downstream of integrin engagement. Over-expression
of dominant-negative FAK causes G1 arrest in mouse fibroblasts
(Zhao et al., 1998). During mitosis, FAK is targeted to the centrosome (Rodriguez-Fernandez et al., 1999), suggesting that FAK may
regulate centrosome functions. This is consistent with the observation that deletion of FAK in endothelial cells leads to multiple
centrosome formation, multi-polar and disorganized mitotic spindles and misalignment of chromosomes during metaphase (Park et
al., 2009).
At the end of mitosis, integrins function to reattach the cells
to its substratum, followed by the establishment of a spread-out
shape and cytoplasmic tension. Similar to cell rounding during
mitosis, these processes are likely regulated by post translational modifications of the attachment proteins. Activation of the
anaphase promoting complex/cyclosome (APC/C) during late mitosis may degrade proteins that target the HEF1, zyxin and other
attachment proteins to mitotic structures, thereby allowing focal
contacts to reform. However, the exact mechanism of focal contact re-establishment and reformation of stress fibers following
cytokinesis is poorly understood. It remains unclear if the formation
of focal contacts precedes cytoskeletal tension or vice versa. Cells
treated with trypsin also show loss of focal contacts and stress fibers
accompanied by cell rounding. However, when replated, these cells
re-establish focal contacts and spreading. Mitotic cells, on the other
hand, stay detached until the completion of mitotic events. It is
likely that cells use distinct mechanisms to promote or inhibit
cytoskeletal events at different stages of cell cycle.
5. Conclusion and perspective
Despite the extensive evidence suggesting that the disruption of
the actin cytoskeleton can lead to cellular arrest, many aspects of
the regulatory relationship between cytoskeletal integrity and cell
cycle progression remain to be elucidated. In particular, it is unclear
if a checkpoint-like mechanism is responsible to ensure the coordination of these two sets of events. More detailed studies of the
direct targets of some key cell cycle regulators such as CDK1, Plk1,
Aurora kinases which are also actin cytoskeleton regulators should
provide further insight into how cell cycle progression can regulate
the actin cytoskeleton and vice versa. Cdc28, the CDK responsible
for cell cycle progression in Saccharomyces cerevisiae and Candida
albicans, has been reported to phosphorylate and regulate the activity of a Rho GEF and IQGAP1, respectively (Kono et al., 2008; Li et
al., 2008). Since CDK1 alone is sufficient to drive cell division in
mammalian cells (Santamaria et al., 2007), it is possible that some
regulatory proteins implicated in maintaining the actin cytoskeleton could be direct targets of CDK1. Many possible CDK1(Cdc28)
targets have been identified in S. cerevisiae (Ubersax et al., 2003)
and in HeLa cells (Blethrow et al., 2008). A thorough analysis of the
roles of these CDK1 targets could lead to the identification of possible regulators of the actin cytoskeleton. Other key effectors, such
as polo kinase, Aurora-A and ubiquitin ligases that play important
roles in mitotic progression, may also serve as critical regulatory
links between the actin cytoskeleton and the mitotic machinery. A
connection between Plk1 and the actin cytoskeleton regulators has
been found in a screen for the Plk1 interactome in U2OS cells at
different stages of the cell cycle (Lowery et al., 2007). The interactome represents partners for a particular protein, isolated through
1631
affinity chromatography and characterized by mass spectrometry.
In this screen, ROCK2 (an effector of RhoA) was found to be an interacting protein of Plk1 as well as being its substrate. Plk1 can control
the local activation of RhoA during cytokinesis and phosphorylate
ROCK2 directly and stimulate ROCK2 activity (Lowery et al., 2007;
Yoshida et al., 2006).
It is very likely that CDK1 plays a central role in integrating the
mechanisms regulating the actin cytoskeleton and mitosis. CDK1,
through its activity on GEFs and GAPs, could affect the activities
of RhoGTPases. These GTPases can further regulate their effector
proteins such as PAK which in turn can regulate Plk1 and Aurora-A
kinases to modulate spindle dynamics. The activities of the RhoGTPases and actin-myosin filaments have been shown to be essential
for spindle formation and for cytokinesis. The same proteins may
be required at different stages of the cell cycle and may function
differently to ensure successful cell division (Fig. 2). A better understanding of the mutual regulatory relationship between the cell
cycle and the actin cytoskeleton will have important implications
for many diseases. This is especially so for cancer biology as many
therapeutic interventions involve interference with cell division of
cancer cells.
Acknowledgements
We thank the Academic Research Fund, MOE Tier 2 and the
Biomedical Research Council, ASTAR Singapore for research funding.
References
Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation
and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem
1996;271:20246–9.
Ando Y, Yasuda S, Oceguera-Yanez F, Narumiya S. Inactivation of Rho GTPases with
Clostridium difficile toxin B impairs centrosomal activation of Aurora-A in G2 /M
transition of HeLa cells. Mol Biol Cell 2007;18:3752–63.
Asiedu M, Wu D, Matsumura F, Wei Q. Centrosome/spindle pole-associated protein
regulates cytokinesis via promoting the recruitment of MyoGEF to the central
spindle. Mol Biol Cell 2009;20:1428–40.
Assoian RK, Zhu X. Cell anchorage and the cytoskeleton as partners in growth factor
dependent cell cycle progression. Curr Opin Cell Biol 1997;9:93–8.
Aszodi A, Hunziker EB, Brakebusch C, Fassler R. Beta1 integrins regulate chondrocyte
rotation, G1 progression, and cytokinesis. Genes Dev 2003;17:2465–79.
Bahmanyar S, Kaplan DD, Deluca JG, Giddings Jr TH, O’Toole ET, Winey M, et al.
beta-Catenin is a Nek2 substrate involved in centrosome separation. Genes Dev
2008;22:91–105.
Bakal CJ, Finan D, LaRose J, Wells CD, Gish G, Kulkarni S, et al. The Rho GTP exchange
factor Lfc promotes spindle assembly in early mitosis. Proc Natl Acad Sci USA
2005;102:9529–34.
Baker J, Garrod D. Epithelial cells retain junctions during mitosis. J Cell Sci
1993;104(Pt 2):415–25.
Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, et al.
p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary
epithelial and cancer cells. J Biol Chem 2004;279:1422–8.
Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell
2007;131:847–60.
Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG.
The formin mDia2 stabilizes microtubules independently of its actin nucleation
activity. J Cell Biol 2008;181:523–36.
Bhadriraju K, Hansen LK. Extracellular matrix dependent myosin dynamics during
G1-S phase cell cycle progression in hepatocytes. Exp Cell Res 2004;300:259–71.
Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic
kinases. Dev Cell 2007;12:699–712.
Blethrow JD, Glavy JS, Morgan DO, Shokat KM. Covalent capture of kinase-specific
phosphopeptides reveals Cdk1-cyclin B substrates. Proc Natl Acad Sci USA
2008;105:1442–7.
Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol 2005;17:123–8.
Brennan IM, Peters U, Kapoor TM, Straight AF. Polo-like kinase controls vertebrate
spindle elongation and cytokinesis. PLoS ONE 2007;2:e409.
Burkard ME, Randall CL, Larochelle S, Zhang C, Shokat KM, Fisher RP, et al. Chemical
genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc Natl Acad Sci USA
2007;104:4383–8.
Canman JC, Lewellyn L, Laband K, Smerdon SJ, Desai A, Bowerman B, et al. Inhibition
of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science
2008;322:1543–6.
1632
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J
Cell Sci 2003;116:435–40.
Carreno S, Kouranti I, Glusman ES, Fuller MT, Echard A, Payre F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization
in mitotic cells. J Cell Biol 2008;180:739–46.
Carroll CW, Altman R, Schieltz D, Yates JR, Kellogg D. The septins are required for
the mitosis-specific activation of the Gin4 kinase. J Cell Biol 1998;143:709–17.
Chakrabarti R, Jones JL, Oelschlager DK, Tapia T, Tousson A, Grizzle WE. Phosphorylated LIM kinases colocalize with gamma-tubulin in centrosomes during early
stages of mitosis. Cell Cycle 2007;6:2944–52.
Cid VJ, Adamikova L, Sanchez M, Molina M, Nombela C. Cell cycle control of
septin ring dynamics in the budding yeast. Microbiology 2001;147:1437–
50.
Clarke DM, Brown MC, LaLonde DP, Turner CE. Phosphorylation of actopaxin regulates cell spreading and migration. J Cell Biol 2004;166:901–12.
Coleman ML, Densham RM, Croft DR, Olson MF. Stability of p21Waf1/Cip1 CDK
inhibitor protein is responsive to RhoA-mediated regulation of the actin
cytoskeleton. Oncogene 2006;25:2708–16.
Croft DR, Olson MF. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and
p27Kip1 levels by distinct mechanisms. Mol Cell Biol 2006;26:4612–27.
Dao VT, Dupuy AG, Gavet O, Caron E, de Gunzburg J. Dynamic changes in Rap1
activity are required for cell retraction and spreading during mitosis. J Cell Sci
2009;122:2996–3004.
den Elzen N, Buttery CV, Maddugoda MP, Ren G, Yap AS. Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian
epithelia. Mol Biol Cell 2009;20:3740–50.
Deng M, Williams CJ, Schultz RM. Role of MAP kinase and myosin light chain
kinase in chromosome-induced development of mouse egg polarity. Dev Biol
2005;278:358–66.
Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002;420:629–35.
Fabian L, Troscianczuk J, Forer A. Calyculin A, an enhancer of myosin, speeds up
anaphase chromosome movement. Cell Chromosome 2007;6:1.
Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus
to the cell cortex. J Cell Biol 1995;131:165–78.
Fielding AB, Dobreva I, McDonald PC, Foster LJ, Dedhar S. Integrin-linked kinase
localizes to the centrosome and regulates mitotic spindle organization. J Cell
Biol 2008;180:681–9.
Fishkind DJ, Cao LG, Wang YL. Microinjection of the catalytic fragment of myosin
light chain kinase into dividing cells: effects on mitosis and cytokinesis. J Cell
Biol 1991;114:967–75.
Forer A, Spurck T, Pickett-Heaps JD. Actin and myosin inhibitors block elongation
of kinetochore fibre stubs in metaphase crane-fly spermatocytes. Protoplasma
2007;232:79–85.
Gachet Y, Reyes C, Goldstone S, Tournier S. The fission yeast spindle orientation checkpoint: a model that generates tension? Yeast 2006;23:1015–
29.
Gachet Y, Tournier S, Millar JB, Hyams JS. A MAP kinase-dependent actin checkpoint
ensures proper spindle orientation in fission yeast. Nature 2001;412:352–5.
Gregory SL, Ebrahimi S, Milverton J, Jones WM, Bejsovec A, Saint R. Cell division
requires a direct link between microtubule-bound RacGAP and Anillin in the
contractile ring. Curr Biol 2008;18:25–9.
Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase
phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci
USA 1992;89:8487–91.
Hara T, Abe M, Inoue H, Yu LR, Veenstra TD, Kang YH, et al. Cytokinesis regulator ECT2
changes its conformation through phosphorylation at Thr-341 in G2 /M phase.
Oncogene 2006;25:566–78.
Hartwell LH. Genetic control of the cell division cycle in yeast. IV. Genes controlling
bud emergence and cytokinesis. Exp Cell Res 1971;69:265–76.
Hazan RB, Kang L, Roe S, Borgen PI, Rimm DL. Vinculin is associated with the Ecadherin adhesion complex. J Biol Chem 1997;272:32448–53.
Herreros L, Rodriguez-Fernandez JL, Brown MC, Alonso-Lebrero JL, Cabanas C,
Sanchez-Madrid F, et al. Paxillin localizes to the lymphocyte microtubule organizing center and associates with the microtubule cytoskeleton. J Biol Chem
2000;275:26436–40.
Hickson GR, O’Farrell PH. Rho-dependent control of anillin behavior during cytokinesis. J Cell Biol 2008;180:285–94.
Hirota T, Morisaki T, Nishiyama Y, Marumoto T, Tada K, Hara T, et al. Zyxin, a regulator
of actin filament assembly, targets the mitotic apparatus by interacting with
h-warts/LATS1 tumor suppressor. J Cell Biol 2000;149:1073–86.
Huang S, Chen CS, Ingber DE. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension.
Mol Biol Cell 1998;9:3179–93.
Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell
adhesion through its direct binding to alpha catenin and actin filaments. J Cell
Biol 1997;138:181–92.
Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the
apical surface during epithelial morphogenesis. J Cell Biol 2008;183:625–33.
Jinguji Y, Ishikawa H. Electron microscopic observations on the maintenance of the
tight junction during cell division in the epithelium of the mouse small intestine.
Cell Struct Funct 1992;17:27–37.
Joo E, Surka MC, Trimble WS. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev Cell 2007;13:677–90.
Kaji N, Muramoto A, Mizuno K. LIM kinase-mediated cofilin phosphorylation
during mitosis is required for precise spindle positioning. J Biol Chem
2008;283:4983–92.
Kimura Y, Morita T, Hayashi K, Miki T, Sobue K. Myocardin functions as an effective
inducer of growth arrest and differentiation in human uterine leiomyosarcoma
cells. Cancer Res 2010;70:501–11.
Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actintemplated assembly of mammalian septins. Dev Cell 2002;3:791–802.
Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, et al. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol
2009;19:1511–8.
Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with
the cadherin/catenin cell–cell adhesion complex via alpha-catenin. J Cell Biol
1995;130:67–77.
Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 2004;6:21–30.
Koh CG. Rho GTPases and their regulators in neuronal functions and development.
Neurosignals 2006;15:228–37.
Kono K, Nogami S, Abe M, Nishizawa M, Morishita S, Pellman D, et al. G1 /S cyclindependent kinase regulates small GTPase Rho1p through phosphorylation of
RhoGEF Tus1p in Saccharomyces cerevisiae. Mol Biol Cell 2008;19:1763–71.
Kremer BE, Adang LA, Macara IG. Septins regulate actin organization and cellcycle arrest through nuclear accumulation of NCK mediated by SOCS7. Cell
2007;130:837–50.
Kunda P, Pelling AE, Liu T, Baum B. Moesin controls cortical rigidity, cell rounding,
and spindle morphogenesis during mitosis. Curr Biol 2008;18:91–101.
Law SF, Zhang YZ, Klein-Szanto AJ, Golemis EA. Cell cycle-regulated processing of
HEF1 to multiple protein forms differentially targeted to multiple subcellular
compartments. Mol Cell Biol 1998;18:3540–51.
Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005;437:275–80.
Lee K, Song K. Actin dysfunction activates ERK1/2 and delays entry into mitosis in
mammalian cells. Cell Cycle 2007;6:1487–95.
Lee YJ, Keng PC. Studying the effects of actin cytoskeletal destabilization on cell cycle
by cofilin overexpression. Mol Biotechnol 2005;31:1–10.
Lew DJ. The morphogenesis checkpoint: how yeast cells watch their figures. Curr
Opin Cell Biol 2003;15:648–53.
Li CR, Wang YM, Wang Y. The IQGAP Iqg1 is a regulatory target of CDK for cytokinesis
in Candida albicans. EMBO J 2008;27:2998–3010.
Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, et al. p21-activated kinase 1
interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep
2002;3:767–73.
Liao L, Jaken S. Effect of alpha-protein kinase C neutralizing antibodies and the pseudosubstrate peptide on phosphorylation, migration, and growth of REF52 cells.
Cell Growth Differ 1993;4:309–16.
Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, et al. Proteomic
screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1
substrate. EMBO J 2007;26:2262–73.
Lu B, Roegiers F, Jan LY, Jan YN. Adherens junctions inhibit asymmetric division in
the Drosophila epithelium. Nature 2001;409:522–5.
Mabuchi I. Biochemical aspects of cytokinesis. Int Rev Cytol 1986;101:175–213.
Mabuchi I, Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol 1977;74:251–63.
Maddox AS, Burridge K. RhoA is required for cortical retraction and rigidity during
mitotic cell rounding. J Cell Biol 2003;160:255–65.
Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell
shape-dependent control of the Skp2-p27kip1 pathway and the G1 /S transition.
J Biol Chem 2004;279:26323–30.
Margadant C, van Opstal A, Boonstra J. Focal adhesion signaling and actin stress
fibers are dispensable for progression through the ongoing cell cycle. J Cell Sci
2007;120:66–76.
Marie H, Pratt SJ, Betson M, Epple H, Kittler JT, Meek L, et al. The LIM protein Ajuba
is recruited to cadherin-dependent cell junctions through an association with
alpha-catenin. J Biol Chem 2003;278:1220–8.
Maroto B, Ye MB, von Lohneysen K, Schnelzer A, Knaus UG. P21-activated kinase is
required for mitotic progression and regulates Plk1. Oncogene 2008;27:4900–8.
Maupin P, Pollard TD. Arrangement of actin filaments and myosin-like filaments in
the contractile ring and of actin-like filaments in the mitotic spindle of dividing
HeLa cells. J Ultrastruct Mol Struct Res 1986;94:92–103.
McMillan JN, Sia RA, Lew DJ. A morphogenesis checkpoint monitors the actin
cytoskeleton in yeast. J Cell Biol 1998;142:1487–99.
Meadows JC, Millar J. Latrunculin A delays anaphase onset in fission yeast by disrupting an Ase1-independent pathway controlling mitotic spindle stability. Mol
Biol Cell 2008;19:3713–23.
Medjkane S, Perez-Sanchez C, Gaggioli C, Sahai E, Treisman R. Myocardin-related
transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat Cell Biol 2009;11:257–68.
Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol
2009;11:71–7.
Minoshima Y, Kawashima T, Hirose K, Tonozuka Y, Kawajiri A, Bao YC, et al. Phosphorylation by Aurora B converts MgcRacGAP to a RhoGAP during cytokinesis.
Dev Cell 2003;4:549–60.
Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity
by regulation of its coactivator MAL. Cell 2003;113:329–42.
Mitsushima M, Toyoshima F, Nishida E. Dual role of Cdc42 in spindle orientation
control of adherent cells. Mol Cell Biol 2009;29:2816–27.
Moulding DA, Blundell MP, Spiller DG, White MR, Cory GO, Calle Y, et al. Unregulated
actin polymerization by WASp causes defects of mitosis and cytokinesis in Xlinked neutropenia. J Exp Med 2007;204:2213–24.
Y.-W. Heng, C.-G. Koh / The International Journal of Biochemistry & Cell Biology 42 (2010) 1622–1633
Nagasaki A, Kanada M, Uyeda TQ. Cell adhesion molecules regulate contractile ringindependent cytokinesis in Dictyostelium discoideum. Cell Res 2009;19:236–46.
Nheu T, He H, Hirokawa Y, Walker F, Wood J, Maruta H. PAK is essential for RASinduced upregulation of cyclin D1 during the G1 to S transition. Cell Cycle
2004;3:71–4.
Niiya F, Tatsumoto T, Lee KS, Miki T. Phosphorylation of the cytokinesis regulator ECT2 at G2 /M phase stimulates association of the mitotic kinase Plk1 and
accumulation of GTP-bound RhoA. Oncogene 2006;25:827–37.
Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation
at the equatorial cortex during cytokinesis. J Cell Sci 2006;119:104–14.
Oceguera-Yanez F, Kimura K, Yasuda S, Higashida C, Kitamura T, Hiraoka Y, et al.
Ect2 and MgcRacGAP regulate the activation and function of Cdc42 in mitosis. J
Cell Biol 2005;168:221–32.
Oegema K, Savoian MS, Mitchison TJ, Field CM. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis.
J Cell Biol 2000;150:539–52.
Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in
cell cycle progression through G1 . Science 1995;269:1270–2.
Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, et al. Overexpression
of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res
1995;55:2752–5.
Park AY, Shen TL, Chien S, Guan JL. Role of focal adhesion kinase Ser-732 phosphorylation in centrosome function during mitosis. J Biol Chem 2009;284:9418–25.
Perez-Moreno M, Song W, Pasolli HA, Williams SE, Fuchs E. Loss of p120 catenin and
links to mitotic alterations, inflammation, and skin cancer. Proc Natl Acad Sci
USA 2008;105:15399–404.
Petronczki M, Glotzer M, Kraut N, Peters JM. Polo-like kinase 1 triggers the initiation
of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to
the central spindle. Dev Cell 2007;12:713–25.
Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, actin, and myosin
during cytokinesis. Curr Biol 2008;18:30–6.
Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem
2002;277:18868–74.
Pradhan D, Lombardo CR, Roe S, Rimm DL, Morrow JS. alpha-Catenin binds directly
to spectrin and facilitates spectrin-membrane assembly in vivo. J Biol Chem
2001;276:4175–81.
Pugacheva EN, Roegiers F, Golemis EA. Interdependence of cell attachment and cell
cycle signaling. Curr Opin Cell Biol 2006;18:507–15.
Reinsch S, Karsenti E. Orientation of spindle axis and distribution of plasma
membrane proteins during cell division in polarized MDCKII cells. J Cell Biol
1994;126:1509–26.
Reshetnikova G, Barkan R, Popov B, Nikolsky N, Chang LS. Disruption of the actin
cytoskeleton leads to inhibition of mitogen-induced cyclin E expression, Cdk2
phosphorylation, and nuclear accumulation of the retinoblastoma proteinrelated p107 protein. Exp Cell Res 2000;259:35–53.
Reverte CG, Benware A, Jones CW, LaFlamme SE. Perturbing integrin function inhibits
microtubule growth from centrosomes, spindle assembly, and cytokinesis. J Cell
Biol 2006;174:491–7.
Rodriguez-Fernandez JL, Gomez M, Luque A, Hogg N, Sanchez-Madrid F, Cabanas C.
The interaction of activated integrin lymphocyte function-associated antigen 1
with ligand intercellular adhesion molecule 1 induces activation and redistribution of focal adhesion kinase and proline-rich tyrosine kinase 2 in T lymphocytes.
Mol Biol Cell 1999;10:1891–907.
Rosenblatt J, Cramer LP, Baum B, McGee KM. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic
spindle assembly. Cell 2004;117:361–72.
Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, et al.
Cdk1 is sufficient to drive the mammalian cell cycle. Nature 2007;448:
811–5.
Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a
structurally distinctive protein-tyrosine kinase associated with focal adhesions.
Proc Natl Acad Sci USA 1992;89:5192–6.
Schmidt A, Durgan J, Magalhaes A, Hall A. Rho GTPases regulate PRK2/PKN2 to
control entry into mitosis and exit from cytokinesis. EMBO J 2007;26:1624–36.
Schroeder TE. Cytokinesis: filaments in the cleavage furrow. Exp Cell Res
1968;53:272–6.
Serra-Pages C, Kedersha NL, Fazikas L, Medley Q, Debant A, Streuli M. The LAR
transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting
protein co-localize at focal adhesions. EMBO J 1995;14:2827–38.
Shafikhani SH, Mostov K, Engel J. Focal adhesion components are essential for mammalian cell cytokinesis. Cell Cycle 2008;7:2868–76.
Shen Y, Schneider G, Cloutier JF, Veillette A, Schaller MD. Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J Biol Chem
1998;273:6474–81.
1633
Spiliotis ET, Kinoshita M, Nelson WJ. A mitotic septin scaffold required for mammalian chromosome congression and segregation. Science 2005;307:1781–5.
Spiliotis ET, Nelson WJ. Here come the septins: novel polymers that coordinate
intracellular functions and organization. J Cell Sci 2006;119:4–10.
Straight AF, Field CM, Mitchison TJ. Anillin binds nonmuscle myosin II and regulates
the contractile ring. Mol Biol Cell 2005;16:193–201.
Sumi T, Hashigasako A, Matsumoto K, Nakamura T. Different activity regulation and
subcellular localization of LIMK1 and LIMK2 during cell cycle transition. Exp Cell
Res 2006;312:1021–30.
Thery M, Bornens M. Cell shape and cell division. Curr Opin Cell Biol 2006;18:648–57.
Thery M, Racine V, Pepin A, Piel M, Chen Y, Sibarita JB, et al. The extracellular matrix
guides the orientation of the cell division axis. Nat Cell Biol 2005;7:947–53.
Toyoshima F, Nishida E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J
2007;26:1487–98.
Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, et al. Targets of
the cyclin-dependent kinase Cdk1. Nature 2003;425:859–64.
Uzbekov R, Kireyev I, Prigent C. Centrosome separation: respective role of microtubules and actin filaments. Biol Cell 2002;94:275–88.
Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, et al. Regulatable
expression of p21-activated kinase-1 promotes anchorage-independent growth
and abnormal organization of mitotic spindles in human epithelial breast cancer
cells. J Biol Chem 2000;275:36238–44.
Wang W, Chen L, Ding Y, Jin J, Liao K. Centrosome separation driven by
actin-microfilaments during mitosis is mediated by centrosome-associated
tyrosine-phosphorylated cortactin. J Cell Sci 2008;121:1334–43.
Wang YL. Dynamics of the cytoskeleton in live cells. Curr Opin Cell Biol
1991;3:27–32.
Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, et al.
alpha-Catenin-vinculin interaction functions to organize the apical junctional
complex in epithelial cells. J Cell Biol 1998;142:847–57.
Watanabe S, Ando Y, Yasuda S, Hosoya H, Watanabe N, Ishizaki T, et al. mDia2 induces
the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol Biol Cell 2008;19:2328–38.
Weiss EE, Kroemker M, Rudiger AH, Jockusch BM, Rudiger M. Vinculin is part of
the cadherin-catenin junctional complex: complex formation between alphacatenin and vinculin. J Cell Biol 1998;141:755–64.
Woolner S, O’Brien LL, Wiese C, Bement WM. Myosin-10 and actin filaments are
essential for mitotic spindle function. J Cell Biol 2008;182:77–88.
Wu X, Kocher B, Wei Q, Hammer III JA. Myosin Va associates with microtubulerich domains in both interphase and dividing cells. Cell Motil Cytoskeleton
1998;40:286–303.
Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherincatenin-actin complex. Cell 2005;123:889–901.
Yamakita Y, Totsukawa G, Yamashiro S, Fry D, Zhang X, Hanks SK, et al. Dissociation
of FAK/p130(CAS)/c-Src complex during mitosis: role of mitosis-specific serine
phosphorylation of FAK. J Cell Biol 1999;144:315–24.
Yamamoto M, Marui N, Sakai T, Morii N, Kozaki S, Ikai K, et al. ADP-ribosylation of
the rhoA gene product by botulinum C3 exoenzyme causes Swiss 3T3 cells to
accumulate in the G1 phase of the cell cycle. Oncogene 1993;8:1449–55.
Yamashiro S, Yamakita Y, Hosoya H, Matsumura F. Phosphorylation of non-muscle
caldesmon by p34cdc2 kinase during mitosis. Nature 1991;349:169–72.
Yasuda H, Kanda K, Koiwa H, Suenaga K, Kidou S, Ejiri S. Localization of actin filaments
on mitotic apparatus in tobacco BY-2 cells. Planta 2005;222:118–29.
Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, et al.
Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature
2004;428:767–71.
Yasuda S, Taniguchi H, Oceguera-Yanez F, Ando Y, Watanabe S, Monypenny J, et
al. An essential role of Cdc42-like GTPases in mitosis of HeLa cells. FEBS Lett
2006;580:3375–80.
Yoshida S, Kono K, Lowery DM, Bartolini S, Yaffe MB, Ohya Y, et al. Polo-like kinase
Cdc5 controls the local activation of Rho1 to promote cytokinesis. Science
2006;313:108–11.
Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol 2005;170:571–82.
Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL. Identification of transcription factor
KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin
D1 and cell cycle progression. Mol Cell 2003;11:1503–15.
Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J
Cell Biol 1998;143:1997–2008.
Zhao WM, Fang G. MgcRacGAP controls the assembly of the contractile ring and the
initiation of cytokinesis. Proc Natl Acad Sci USA 2005;102:13158–63.
Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to
the centrosome and regulates Aurora-A. Mol Cell 2005;20:237–49.