Determination of the Rate of a Reaction,
Its Order, and Its Activation Energy
Reaction kinetics is defined as the study of the rates of chemical reactions and their mechanisms. Reaction
rate is simply defined as a change in a measurable quantity divided by the change in time. In chemistry, the
“measurable quantity” is usually molar concentration or absorbance. Consider the generalized chemical
reaction equation A + B → C + D. Symbolically it can be represented in multiple ways:
Rate = −
Δ [A]
= k[A]m
Δ time
Note that the units on rate are always M/time which can also be expressed as M time−1. The negative sign
on the first expression indicates that the molar concentration of reactant A will decrease as time goes by.
The second expression is simply the differential rate law expression where the rate constant k, and the order
of reactant A (the exponent m) must be experimentally determined. Never, ever forget that the value of k is
temperature dependent. Since two reactants are present in our example reaction we can write comparable
expressions for reactant B, but beware that the order of B will not necessarily be the same as the order for A,
so we often use a different variable, such as n, for the exponent on B.
The differential rate law can be integrated to link changes in concentration with time as opposed to rate. This
sounds way more complicated that it really is! (“Integrated” is a Calculus term you need not worry about in
this course—we will linearlize the data to avoid Calculus since it is not a prerequisite for AP Chemistry.)
In this experiment you will investigate the reaction of crystal violet with sodium hydroxide. Crystal violet,
in aqueous solution, is often used as an indicator in biochemical testing. The reaction of this organic molecule
with sodium hydroxide can be simplified by abbreviating the chemical formula for crystal violet as CV.
CV+(aq) + OH–(aq) → CVOH(aq)
As the reaction proceeds, the violet-colored CV+ reactant will slowly change to a colorless product, following
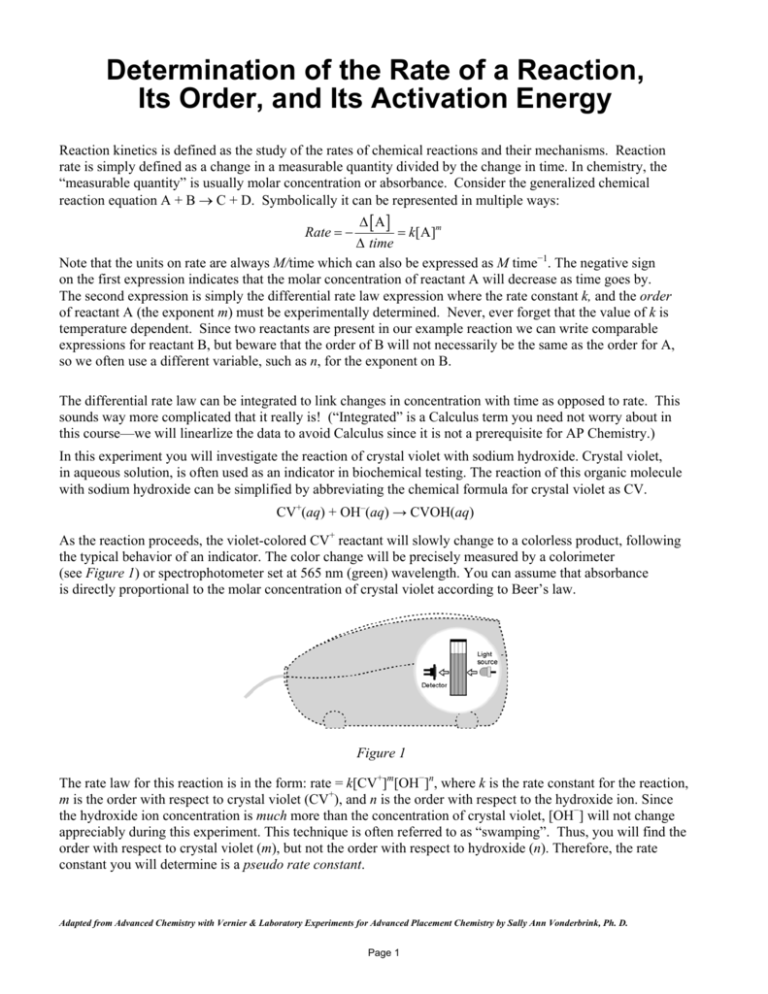
the typical behavior of an indicator. The color change will be precisely measured by a colorimeter
(see Figure 1) or spectrophotometer set at 565 nm (green) wavelength. You can assume that absorbance
is directly proportional to the molar concentration of crystal violet according to Beer’s law.
Figure 1
The rate law for this reaction is in the form: rate = k[CV+]m[OH−]n, where k is the rate constant for the reaction,
m is the order with respect to crystal violet (CV+), and n is the order with respect to the hydroxide ion. Since
the hydroxide ion concentration is much more than the concentration of crystal violet, [OH−] will not change
appreciably during this experiment. This technique is often referred to as “swamping”. Thus, you will find the
order with respect to crystal violet (m), but not the order with respect to hydroxide (n). Therefore, the rate
constant you will determine is a pseudo rate constant.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 1
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
You will use integrated rate law methods to determine the order m and the value of the rate constant by
graphing the absorbance and time data that you collect. Set up your axes so that time is always on the x-axis.
Plot the absorbance of CV+ on the y-axis of the first graph. Plot the natural log of the absorbance of CV+
(ln [CV+], NOT log[CV+]) on the y-axis of the second graph and the reciprocal of the absorbance of CV+ on
the y-axis of the third graph. You are in search of the best linear fit. Here comes the elegant part… If you do
the set of graphs in this order with the y-axes being “concentration”, “natural log of concentration” and
“reciprocal concentration”, the alphabetical order of the y-axis variables leads to orders of 0, 1 and 2
respectively for CV+. You can then quickly derive the integrated rate law equations using y = mx + b.
Zero order
k = negative slope
First order
k = negative slope
Second order
k = the slope
Another important part of the kinetic analysis of a chemical reaction is to determine the activation energy, Ea.
Activation energy can be defined as the energy necessary to initiate an otherwise spontaneous chemical
reaction so that it will continue to react without the need for additional energy. An example of activation
energy is the combustion of paper. The reaction of cellulose and oxygen is spontaneous, but you need to
initiate the combustion by adding activation energy from a lit match.
We can use a different graphical analysis method to easily determine the activation energy of a chemical
reaction. Each laboratory group will simply repeat the reaction between crystal violet and sodium hydroxide at
a temperature other than room temperature, while keeping the initial concentrations of the reactants the same
for each trial. Recall that the value of k is temperature dependent. Class data will be collected, graphed and
analyzed as follows:
Ea = −R × slope
ln k
1 −1
K
T
( )
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 2
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
OBJECTIVES
In this experiment, you will
• React solutions of crystal violet and sodium hydroxide at different temperatures.
• Graph the concentration-time data and use integrated rate law methods to determine the order of CV
and the value of a pseudo rate constant, k, for the reaction.
• Measure and record the effect of temperature on the reaction rate and rate constant.
• Calculate the activation energy, Ea, for the reaction.
Figure 1
MATERIALS
Data collection device
computer or handheld
colorimeter or spectrophotometer
temperature probe or thermometer
cups or beakers
ice or hot water bath
0.10 M NaOH solution
2.5 × 10–5 M crystal violet solution
plastic cuvettes
Beral pipettes
PROCEDURE
1. Obtain and wear goggles.
2. Set up the data collection system.
a. Calibrate your spectrophotometer or your colorimeter. We will be collecting data using the 565 nm
(Green) setting.
b. Connect a temperature probe to your device.
c. If using a spectrophotometer, you will need to manually record data every 5 seconds. If using a
colorimeter set the program to generate a time graph with 3 seconds between samples for 60 samples.
Do NOT start data collection until Step 3 b.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 3
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
3. This first trial will be performed at room temperature. You have been given a pipette of 0.10 M NaOH
(colorless solution) and a pipette containing an equal quantity of 2.5 × 10–5 M crystal violet (purple
solution).
a. Simultaneously squirt both solutions into a beaker or cup. Use the tip of the temperature probe to stir
the mixture. Record the temperature of the mixture.
b. Rinse the cuvette with the mixture, discard the rinse into the sink, refill the cuvette at least 2/3 full and
place it correctly in the colorimeter and start data collection for the first trial.
c. Once the trial is finished, discard the reaction mixture into the sink.
4. Analyze your data using graphical methods as explained in the introduction. Determine the order of CV+
and value of the rate constant, k. Record the value of the rate constant for this trial before proceeding to the
next step.
5. Repeat Steps 3-4, using the second set of pipettes that have been sitting in the water bath at your station.
6. Record your data in the class data table that your teacher has displayed. For your own lab report, mark your
group number with an asterisk *.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 4
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
CLASS DATA TABLE
Trial 1
Rate constant, k
Room Temperature
(supply appropriate units)
(°C)
Trial 2
Temperature
(°C)
Rate constant, k
(supply appropriate units)
Group 1
Group 2
Group 3
Group 4
Group 5
Group 6
Group 7
Group 8
Group 9
Group 10
Group 11
Group 12
Group 13
Group 14
Group 15
Group 16
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 5
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
PRE-LAB QUESTIONS
1. Refer back to generalized chemical reaction presented in the introduction and write two comparable rate
expressions for reactant B.
2. Compare the molar concentration of the crystal violet solution to that of the sodium hydroxide solution.
Approximately how much more concentrated is the sodium hydroxide? Justify your answer.
3. Why do we set the spectrophotometer or colorimeter to a wavelength of 565 nm or “green” in order to
measure the absorbance of crystal violet?
4. A student mixes 3.00 mL of 2.27 × 10−5 M crystal violet solution with 3.00 mL 0.1 M sodium hydroxide
both at 24.5 °C and collects the following data:
Time
(min)
1.49
4.17
6.22
8.15
10.10
13.00
14.02
16.18
19.00
Absorbance
0.206
0.157
0.131
0.108
0.093
0.072
0.066
0.055
0.044
(a) Describe the graphical analysis steps the student should perform in order to determine the
(i)
Order of the reaction with respect to crystal violet
(ii)
Value of the rate constant, k
(b) Use a graphing calculator or computer graphing software to determine the order of the reaction with
respect to crystal violet. Justify your answer.
(c) Write the law expression for this reaction. Justify your answer.
(d) Determine the value of k including its units. Justify your answer.
(e) Calculate the half –life of the reaction. Include units with your answer.
(f) Determine the absorbance of crystal violet at 3.00 minutes. Justify your answer.
(g) Determine the time at which the absorbance of crystal violet is equal to 0.060.
5. The student repeats the experiment at 32.5 °C with the same initial quantities and molarities of crystal
violet and sodium hydroxide. Predict whether the value of the rate constant k will be increase, decrease or
remain unchanged. Justify your answer.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 6
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
POST-LAB QUESTIONS AND DATA ANALYSIS
1. Graph the class data and calculate the activation energy, Ea, for the crystal violet and sodium hydroxide
reaction.
2. Extrapolate your graph to predict the value of the rate constant k for this reaction at 40 °C.
3. A well-known approximation in chemistry states that the rate of a reaction often doubles for every
10°C increase in temperature. Use your data to verify or refute this approximation.
4. A student failed to fill the cuvette 2/3 full with the reaction mixture. What effect does this error have on
the measured absorbance values?
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 7
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
TEACHER INFORMATION
1. This experiment conforms to the guidelines for the twelfth laboratory experiment listed in the
College Board AP Chemistry guide (the Acorn book). It is best to perform this laboratory exercise
after students have worked problems involving both integrated rate law and the Arrhenius equation.
2. Any 0.10 M NaOH solution will work for this lab since the solution need not be quantitatively
prepared. Dissolve 0.8 g solid NaOH in DI water to make 200 mL of solution. Since this lab is
microscale, 200 mL should be more than enough solution.
3. Crystal violet (C25H30CIN3 with a molar mass of 408.00 g/mol) will leave stains on skin and clothing,
so wear an apron, goggles and gloves. Dissolve 0.0020 g of CV in DI water to make 200 mL of
solution. If you do not have an analytical balance, you can dissolve 0.20 g CV in DI water to make
2 L of solution, and then dilute 100 mL of this solution to a total volume of 1 L. Store CV in a brown
or foil-covered bottle and refrigerate if possible. 1 M HCl will remove CV stains from glassware.
4. Students can collect data very quickly if you prepare Beral pipettes of both reactant solutions ahead of
time. Draw up about 3-5 mL of each solution into the pipettes. It is not important that you measure the
volume of the solutions accurately since the NaOH solution is about 10,000× more concentrated than
the crystal violet solution. You simply want approximately equal volumes of each reactant and enough
total solution volume so that students can rinse their cuvette with the new reaction solution between
trials and have enough to fill the cuvette about 2/3 full.
5. Place a NaOH and crystal violet (CV) pipette at each station prior to the lab period for the room
temperature trial. Set up an ice bath and a hot water bath (no need to boil the water) with matched sets
of NaOH and CV pipettes so that students can conduct a second trial at a different temperature.
6. The time necessary for data collection of a given trial during this lab is very quick. Students squirt both
reactants into a small beaker or cup, stir with their temperature probe, record the temperature, rinse
their cuvette with the reacting mixture, fill the cuvette 2/3 full and place it into the colorimeter or
spectrophotometer and collect data for 180 seconds. Caution students to work quickly once they have
recorded the initial temperature of the reaction mixture. Failure to do so will result in the plot of
ln k vs. 1/T not being perfectly linear.
HAZARD ALERTS
Sodium Hydroxide: Corrosive solid; skin burns are possible; much heat evolves when added to water; very
dangerous to eyes; wear face and eye protection when using this substance. Wear gloves. Hazard Code:
B—Hazardous.
Crystal Violet: Moderately toxic by ingestion; body tissue irritant. Hazard Code: C—Somewhat hazardous.
The hazard information reference is: Flinn Scientific, Inc., Chemical and Biological Catalog Reference
Manual, P.O. Box 219, Batavia, IL 60510, (800) 452-1261, www.flinnsci.com
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 8
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
SAMPLE DATA TABLE
Trial 1
Rate constant, k
Room Temperature
(supply appropriate units)
Trial 2
Temperature
(°C)
(supply appropriate units)
0.006104
10.5
0.002689
Group 2
16.3
0.004577
Group 3
24.4
0.007441
(°C)
Group 1*
20.8
Rate constant, k
* When calculating the Ea students should use their own room temperature data along with however
many data points the class collects at temperatures other than room temperature.
Answers to PRE-LAB QUESTIONS
1. Refer back to generalized chemical reaction presented in the introduction and write two comparable rate
expressions for reactant B.
Rate = −
Δ [ B]
Δ time
= k[B]m
2. Compare the molar concentration of the crystal violet solution to that of the sodium hydroxide solution.
Approximately how much more concentrated is the sodium hydroxide? Justify your answer.
The sodium hydroxide solution is approximately 10,000 times more concentrated than the crystal violet
solution. The two molar concentrations differ by a factor of ≈10−4 since the molarity of crystal violet is
2.5 × 10−5 versus a molarity of 1.0 × 10−1 for the sodium hydroxide solution.
3. Why do we set the spectrophotometer or colorimeter to a wavelength of 565 nm or “green” in order to
measure the absorbance of crystal violet?
The three colors of primary light are red, green and blue. We see CV as purple, since it is reflecting a
mixture of red and blue light, thus absorbing green light. Therefore, CV will have maximum absorbance
values in the region of the visible spectrum that corresponds to green light.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 9
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
4. A student mixes 3.00 mL of 2.27 × 10−5 M crystal violet solution with 3.00 mL 0.100 M sodium
hydroxide both at 24.5 °C and collects the following data:
Time
(min)
1.49
4.17
6.22
8.15
10.10
13.00
14.02
16.18
19.00
Absorbance
0.206
0.157
0.131
0.108
0.093
0.072
0.066
0.055
0.044
(a) Describe the graphical analysis steps the student should perform in order to determine the
(i) Order of the reaction with respect to crystal violet
The student should create a new data column for ln absorbance (abs) and a second new data
column for absorbance−1. Next, three graphs should be generated: abs vs. time, ln abs vs. time
and abs−1 vs. time. The graph with the linear regression correlation coefficient, r closest to the
absolute value of 1.0 has the best linear fit for the data and thus indicates the order of the
reaction with respect to crystal violet. If the abs vs. time graph is the best linear fit, the order
for CV is zero. If the ln abs vs. time graph is the best linear fit, the order for CV is 1st. If the
abs−1 vs. time graph is the best linear fit, then the order for CV is 2nd.
(ii) Value of the rate constant, k
The value of k = slope of the line with the best linear fit as explained in part (i) above.
(b) Use a graphing calculator or computer graphing software to determine the order of the reaction with
respect to crystal violet. Justify your answer.
The reaction is first order. This graphing calculator
screen shot shows the three
absorbance columns (abs given = L2, ln abs = L3
and abs−1 = L4). The next step is to determine the
r-value for each of these data columns vs. time.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 10
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
absorbance vs. time (L1, L2)
r = 0.9677
ln absorbance vs. time (L1, L3)
r = 0.9993
absorbance−1 vs. time (L1, L4)
r = 0.9826
∴ The best linear fit is indicated by the r-value closest to ±1 which is the graph of ln absorbance vs. time
which means the reaction is first order. In this case, the determination could have been made by
inspecting the graphs. It is clear that the first and third graphs are curves, but that is not always obvious.
Students should be encouraged to calculate the r-values or “correlation” values as verification.
(c) Write the law expression for this reaction. Justify your answer.
rate = k[CV+] (students may elect to put an exponent of “1” if they wish) since the graph of
ln absorbance vs. time yields the most linear fit.
(d) Determine the value of k including its units. Justify your answer.
k = slope for the ln absorbance vs. time graph shown
center above ∴ k = 0.0877 min−1 or
k = 8.87 × 10−2 min−1
(e) Calculate the half –life of the reaction. Include units with your answer.
Students should review the data table and note that at t = 1.49
min, abs = 0.206, so solving for time when abs = 0.103, then
subtracting from the original time of 1.49 min will give the halflife of the reaction. The complicating factor is that they have to
remember they graphed ln abs, not just abs! Next, they press
yr· followed by ÍÍÍ to obtain the time
at which the abs has been reduced by ½ which is equal to 9.02
minutes. Therefore it took 9.02 – 1.49 minutes for the
absorbance to be reduced by ½, so t1/2 = 7.53 minutes. Answers
may vary a bit if students chose other data points, but
reasonable answers fall between 7 and 8 minutes.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 11
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
(f) Determine the absorbance of crystal violet at 3.00 minutes. Justify your answer.
Students may elect to solve for the absorbance value
graphically or algebraically.
Graphically: The student should display the graph of ln
absorbance vs. time. Next they should evaluate the y-value that
corresponds to a time of 3.00 minutes. The first screen shot on
the following page was obtained by graphing L1, L3 along with
the regression line in Y1 and pressing yr (that places
“X=” on the screen) and entering 3.00. Pressing enter, solves
the regression equation and stores the ln absorbance value as
“Y” which is shown in the second screen shot below. Pressing
yz to quit the graph and yμƒÀ to solve for the
anti natural log of “Y” gives a value of 0.1746 M which is
reasonable compared to the actual data points in the data table.
Algebraically:
Students should derive the y = mx + b form of the 1st order integrated rate law (from their graph) as:
ln ⎡⎣CV + ⎤⎦ = kt + ln ⎡⎣CV + ⎤⎦
0
⎛ 0.0877 ⎞
ln ⎡⎣CV + ⎤⎦ = ⎜ −
⎟ 3.00 min + ln [ 0.227 ]0
⎝ min ⎠
ln ⎡⎣CV + ⎤⎦ = −0.2631 + ( −1.483) = −1.7461
(
)
∴ ⎡⎣CV + ⎤⎦ = e −1.7461 = 0.1745M
(g) Determine the time at which the absorbance of crystal violet is equal to 0.060.
Again, students may solve graphically or algebraically, whichever is fastest and most accurate for
them.
Graphically:
Begin by placing ln(0.060) into Y2 and pressing s to display the graph. You should see the
intersection of the regression equation, and the straight horizontal line that is the graph of ln(0.060).
(If ever you don’t see the intersection, press p and select “Zoom Out” then press Í.)
Next, press yr· followed by ÍÍÍ to obtain the x-value that corresponds to
the time of 15.18 minutes.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 12
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
Algebraically:
ln ⎡⎣CV + ⎤⎦ = − kt + ln ⎡⎣CV + ⎤⎦
0
∴ −kt = ln ⎣⎡CV ⎦⎤ − ln ⎣⎡CV ⎦⎤
0
+
+
( ln ⎡⎣CV ⎤⎦ − ln ⎡⎣CV ⎤⎦ )
∴t =
+
+
0
∴t =
−k
( ln [0.060] − ln [0.227])
−0.0877 min −1
= 15.17 min
5. The student repeats the experiment at 32.5 °C with the same initial quantities and molarities of crystal
violet and sodium hydroxide. Predict whether the value of the rate constant k will be increase, decrease or
remain unchanged. Justify your answer.
The value of k should increase. Increasing the temperature increases the average kinetic energy of the
molecules which also increases the number of molecules having sufficient energy to overcome the
activation energy. Additionally, collision frequency increases as does the number of effective collisions.
Answers to POST-LAB QUESTIONS AND DATA ANALYSIS
1. Graph the class data and calculate the activation energy, Ea, for the crystal violet and sodium hydroxide
reaction.
The screen shot on the left above shows the sample data as given where L1 contains the temperature
in °C and L2 contains the corresponding values of the rate constant.
Students should batch transform the data by going to the very top of the L1column and press
£yÀÃÁ¬ÂËÀ·¤— to convert the Celsius temperatures to reciprocal Kelvin temperatures.
Next, go to the very top of the L2 column and press μyÁ to calculate the natural logs of the k values.
The results are shown in the second screen shot above.
The next step is to set up a graph plotting ln k vs. 1/T and perform a linear regression analysis to obtain the
value of the slope. Those results are shown in the thrid and fourth screen shots above.
⎛
⎞
J
J
kJ
Therefore, Ea = − R × slope = − ⎜ 8.31
or 51.4
⎟ −6186 K = 51, 400
mol
mol
mol• K ⎠
⎝
(
)
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 13
Determination of the Rate of a Reaction, Its Order and Its Activation Energy
2. Extrapolate your graph to predict the value of the rate constant k for this reaction at 40 °C.
Students will have to press q (Zoom Out) Í to resize their graph. Once resized, they can press
yrÀ to enter the x-value, remembering they graphed temperatures as reciprocal Kelvins. Pressing
Í gives a y-value that is equal to the ln of k and stored in the calculator as “Y”. Pressing yz to
quit the graph followed by pressing yμ and then ƒÀ to paste y-value shown on the second
screen shot above to obtain the antilog which is 0.02178 as shown on the third screen shot above.
Students should report an answer with correct units, so k = 0.0218 s−1.
3.
A well-known approximation in chemistry states that the rate of a reaction often doubles for every
10°C increase in temperature. Use your data to verify or refute this approximation.
0.006104
= 2.27
0.002689
which verifies the approximation. It is important to note that this approximation depends on the activation
energy for the reaction as well.
Comparing the 10.5 °C sample data trial to the 20.8 °C trial, the ratio of the k values is
4. A student failed to fill the cuvette 2/3 full with the reaction mixture. What effect does this error have on
the measured absorbance values?
Student answers will vary dependent upon your colorimetric equipment. It could be that there would be no
effect if the light path is positioned low enough so that all of the light passes through the volume of the
sample. If the light path is higher than the 2/3 mark on the cuvette, then light not passing through the
sample strikes the photodetector unabsorbed which significantly lowers the measured absorbance values.
Adapted from Advanced Chemistry with Vernier & Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph. D.
Page 14
2004 AP® CHEMISTRY FREE-RESPONSE QUESTIONS
3. The first-order decomposition of a colored chemical species, X, into colorless products is monitored with a
spectrophotometer by measuring changes in absorbance over time. Species X has a molar absorptivity constant
of 5.00 × 103 cm–1 M –1 and the path length of the cuvette containing the reaction mixture is 1.00 cm. The data
from the experiment are given in the table below.
[X]
(M)
?
4.00 × 10 – 5
3.00 × 10 – 5
1.50 × 10 – 5
Absorbance
0.600
0.200
0.150
0.075
Time
(min)
0.0
35.0
44.2
?
(a) Calculate the initial concentration of the colored species.
(b) Calculate the rate constant for the first-order reaction using the values given for concentration and time.
Include units with your answer.
(c) Calculate the number of minutes it takes for the absorbance to drop from 0.600 to 0.075.
(d) Calculate the half-life of the reaction. Include units with your answer.
(e) Experiments were performed to determine the value of the rate constant for this reaction at various
temperatures. Data from these experiments were used to produce the graph below, where T is temperature.
This graph can be used to determine the activation energy, Ea , of the reaction.
(i) Label the vertical axis of the graph.
(ii) Explain how to calculate the activation energy from this graph.
STOP
If you finish before time is called, you may check your work on this part only.
Do not turn to the other part of the test until you are told to do so.
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
8
Page 15
GASES, LIQUIDS, AND SOLUTIONS
PV = nRT
Ê
n2 a ˆ
ÁË P + 2 ˜¯ (V - nb) = nRT
V
PA = Ptotal ¥ X A , where X A =
Ptotal = PA + PB + PC + ...
m
n=
M
moles A
total moles
K = D C + 273
PV
PV
1 1
= 2 2
T1
T2
m
D=
V
3kT
3 RT
urms =
=
m
M
1 2
KE per molecule = mu
2
3
KE per mole = RT
2
r1
M2
=
r2
M1
molarity, M = moles solute per liter solution
molality = moles solute per kilogram solvent
DT f = iK f ¥ molality
DTb = iK b ¥ molality
p = iMRT
A
Beer's Law: = abc
P
V
T
n
D
m
u
=
=
=
=
=
=
=
pressure
volume
temperature
number of moles
density
mass
velocity
urms
KE
r
M
p
i
Kf
=
=
=
=
=
=
=
root-mean-square speed
kinetic energy
rate of effusion
molar mass
osmotic pressure
van't Hoff factor
molal freezing-point depression constant
Kb
A
a
b
c
Q
I
q
t
=
=
=
=
=
=
=
=
=
molal boiling-point elevation constant
absorbance
molar absorptivity
path length
concentration
reaction quotient
current (amperes)
charge (coulombs)
time (seconds)
E D = standard reduction potential
K = equilibrium constant
OXIDATION-REDUCTION; ELECTROCHEMISTRY
Gas constant, R = 8.31 J mol -1 K -1
= 0.0821 L atm mol -1 K -1
Q=
I =
[C] c [D] d
a
[A] [B]
= 62.4 L torr mol -1 K -1
, where a A + b B Æ c C + d D
= 8.31 volt coulomb mol -1 K -1
Boltzmann's constant, k = 1.38 ¥ 10 -23 J K -1
q
t
D Ecell = Ecell
log K =
b
K f for H 2 O = 1.86 K kg mol -1
RT
D - 0.0592 log Q @ 25DC
ln Q = Ecell
nᏲ
n
K b for H2 O = 0.512 K kg mol -1
1 atm = 760 mm Hg
= 760 torr
nE D
0.0592
STP = 0.00 D C and 1.0 atm
Faraday's constant, Ᏺ = 96,500 coulombs per mole
of electrons
GO ON TO THE NEXT PAGE.
Page 16
AP® CHEMISTRY
2004 SCORING GUIDELINES
Question 3
The first-order decomposition of a colored chemical species, X, into colorless products is monitored with a
spectrophotometer by measuring changes in absorbance over time. Species X has a molar absorptivity
constant of 5.00 × 103 cm–1 M –1 and the path length of the cuvette containing the reaction mixture is 1.00 cm.
The data from the experiment are given in the table below.
[X]
(M)
?
4.00 × 10 – 5
3.00 × 10 – 5
1.50 × 10 – 5
Absorbance
0.600
0.200
0.150
0.075
Time
(min)
0.0
35.0
44.2
?
(a) Calculate the initial concentration of the colored species.
A = abc
c =
A
=
ab
0.600
3
−1
×
5.00
10
cm
M −1 ) (1.00 cm )
(
= 1.20 × 10− 4 M
A0 = abc0
A 0 A1
=
c0
c1
OR
1 point for concentration of X
A1 = abc1
0.600
0.200
=
c0
4.00 × 10−5
c0 = 1.20 × 10− 4 M
(b) Calculate the rate constant for the first-order reaction using the values given for concentration and time.
Include units with your answer.
Using the first two readings,
ln[X]t – ln[X]0 = −kt
ln
4.00 × 10−5
1.20 × 10−4
OR
ln
[X]t
= −kt
[X]0
= −k (35.0 min)
1 point for magnitude and correct sign of rate constant
ln (0.333) = −k (35.0 min)
1 point for correct units
–1.10 = −k (35.0 min)
k = 3.14 × 10−2 min−1
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
7
Page 17
AP® CHEMISTRY
2004 SCORING GUIDELINES
Question 3 (cont’d.)
(c) Calculate the number of minutes it takes for the absorbance to drop from 0.600 to 0.075.
ln
ln
[X]t
= −k t
[X]0
1.50 × 10−5
1.20 × 10
−4
1 point for correct substitution
= (−3.14 × 10−2 min−1) t
1 point for correct answer
ln (0.125) = (−3.14 × 10−2 min−1) t
–2.08 = (−3.14 × 10−2 min−1) t
t = 66.2 min
Note: students may use half-lives to answer this question.
(d) Calculate the half-life of the reaction. Include units with your answer.
ln
[X]t
= −kt
[X]0
ln
0.5 [X]0
= (−3.14 × 10−2 min−1) t1/2
[X]0
1 point for correct magnitude
ln (0.5) = (−3.14 × 10−2 min−1) t1/2
1 point for the correct units
−0.693 = (−3.14 × 10−2 min−1) t1/2
22.1 min = t1/2
OR
t1/2
0.693
=
k
t1/2 =
(1 point for the half-life equation if no k is given)
0.693
= 22.1 min
3.14 × 10−2 min −1
(e) Experiments were performed to determine the value of the rate constant for this reaction at various
temperatures. Data from these experiments were used to produce the graph below, where T is
temperature. This graph can be used to determine the activation energy, Ea , of the reaction.
(i) Label the vertical axis of the graph.
The vertical axis should be labeled ln k.
1 point
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
8
Page 18
AP® CHEMISTRY
2004 SCORING GUIDELINES
Question 3 (cont’d.)
(ii) Explain how to calculate the activation energy from this graph.
The slope of the line is related to the activation energy:
1 point for recognizing that the slope must be
measured
E
slope = – a
R
1 point for the correct explanation of how to obtain
the activation energy
To determine the activation energy for the reaction,
multiply the slope by – 8.314 J mol−1 K−1.
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
9
Page 19
Page 20
Page 21
AP® CHEMISTRY
2004 SCORING COMMENTARY
Question 3
Sample: 3A
Score: 10
This response earns a perfect score of 10 points: 1 point for part (a), 2 points for part (b), 2 points for part (c),
2 points for part (d), 1 point for part (e)(i), and 2 points for part (e)(ii).
Sample: 3B
Score: 8
This response demonstrates two common errors: only 1 out of 2 points is earned in part (b) because
incorrect units are used for the rate constant, and only 1 out of 2 points is earned in part (d) because there
are no units for the half-life.
Sample: 3C
Score: 7
The point is not earned in part (e)(i) because the vertical axis is labeled ln[A] instead of ln k, a common
error. No points are earned in part (e)(ii) because the answer does not say anything about the slope or how
it is related to activation energy.
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for students and parents).
4
Page 22
2004 Q&A
Question 3
What was the intent of this question?
This question tested students’ ability to use data from a first-order kinetics experiment to determine the
rate constant; determine the half-life; relate the concentration of the reactant to the time of the reaction;
use the Arrhenius equation to calculate the activation energy.
How well did students perform on this question?
Approximately 7 percent of the students chose this question. The average score was 1.7 out of a possible
10 points. Points were most often earned in Parts (a), (c), and (d).
What were common student errors or omissions?
Part (a): Most of the students were able to correctly determine the concentration of the colored species, X,
at an absorbance of 0.600. Students were split between two methods of determining the initial
concentration of the colored species. Beer’s Law was correctly used by most students who decided to use
that method (this was the most error-free method).
4
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
Page 23
Many students used a proportionality approach, relating the different absorbances at 0.0 minutes and 35.0
minutes to the respective concentrations of X. Errors were common with this method: students set up the
proportionality incorrectly, and they made numerous algebra mistakes.
Another common mistake centered around incorrectly changing the determined initial concentration of
12.0 × 10−5 M to 1.20 × 10−6 M.
Part (b): Problems arose when the students were unable to select the correct expression for the rate law
from the given equations sheet. Of those who did select the correct expression, most were able to handle
the natural logarithm calculation. A common mistake arose in the final sign of the rate constant. Many
students gave a negative value, due to an algebra error. Frequently, one point was earned for the correct
magnitude of the rate constant.
The second point of Part (b) was awarded if the correct units for the rate constant were given. This point
was rarely earned. Many students included molarity (in many different ways) in their units.
Parts (c) and d): These sections were difficult to grade. Readers found it best to read both c) and d) before
determining how many points had been earned. Students were asked to determine the number of minutes
required to have the absorbance drop from 0.600 to 0.075 and to determine the half-life of the reaction.
Methodology was equally split: Some students used the first-order integrated rate law expression, and
some used a method based on inspection of the data.
Students who opted to use the first-order integrated rate law expression usually earned full credit for both
parts. Students who answered by inspection of the data and saw that there were two half-lives from 0.0
minutes to 44.2 minutes usually got the correct answer for the half-life. This half-life value was then
added to the 44.2 minutes at an absorbance of 0.150 to give 66.2 minutes at the absorbance of 0.075
(another half-life). This approach led to numerous errors. Many students incorrectly determined the halflife by simply taking 44.2 and dividing by 2. This did not earn any points if it was unclear whether the 2
meant 2 half-lives or 2 = 0.150/0.075. The student needed to be clear on how this value was determined.
A point was also awarded for the correct units for the half-life. Many students earned this point.
Part (e)(i): Students were asked to label the vertical axis of the graph provided. This was the most
commonly missed point. Incorrect responses included [X], ln[X], absorbance, and 1/[X].
Part (e)(ii): Again, these were a very difficult two points for many students. From their answers, it was
clear that many knew that the graph represents the following relationship:
ln k =
()
− Ea 1
+ ln A
R T
However, many students were not able to explain how to determine the activation energy from the graph.
Many took an algebraic approach to describe what variables were known and how the equation had to be
manipulated to get a value for Ea.
Based on your experience of student responses at the AP Reading, what message would you like to
send to teachers that might help them to improve the performance of their students on the exam?
•
•
Instructors need to show more varied types of kinetics problems.
Describe to students the characteristics of zero-, first-, and second-order reactions. Students
should be able to answer the following:
• How are graphs used to describe each type of relationship?
• How are the rate constant and its units determined?
• How may the equation be used to determine unknown concentrations?
• How is the half-life determined?
5
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
Page 24
Encourage your students to read questions carefully. Many students did not follow the instruction
for part (e)(ii), “Explain how to calculate the activation energy from this graph.”
•
Repeated emphasis needs to be given to describing how a graphical representation of an equation
may be used to obtain useful information.
•
Finally, if units are asked for in a problem, then the units will earn points. There were many good
papers where the units were completely missing.
Question 4
What was the intent of this question?
This question was designed to assess students’ familiarity with chemical nomenclature and knowledge of
common classes of chemical reactions, as well as their ability to apply their knowledge in predicting the
products(s) of a variety of chemical reactions.
How well did students perform on this question?
The question asked students to choose five of eight reactions. The most popular choices were the
displacement of copper ions from solution by aluminum metal (a), the acid/base reaction (c), the
precipitation of aluminum phosphate (g), and the production of hydrogen sulfide (h). The mean score
was 6.7 out of a possible 15 points.
What were common student errors or omissions?
Reaction (a):
• Failing to dissociate the copper(II) sulfate.
• Producing aluminum sulfate as a product.
• Assigning a charge of +2 to the aluminum ion.
• Reversing the reaction.
Reaction (b):
• Failing to give the correct formula for dimethyl ether.
Reaction (c):
• Failing to represent nitrous acid in the molecular form.
• Writing a double-displacement reaction using non-dissociated compounds.
• Failing to place a negative charge on the nitrite ion.
• Confusing nitrous acid with nitric acid.
Reaction (d):
• Writing gaseous hydrogen iodide as H+.
• Writing molecular carbonic acid as a product instead of water and carbon dioxide.
• Writing a double-displacement reaction using compounds.
• Failing to dissociate lithium carbonate.
• Producing lithium iodide and HCO3 as compounds in solution.
6
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
Page 25