ELEMENTS, COMPOUNDS, AND MIXTURES
advertisement

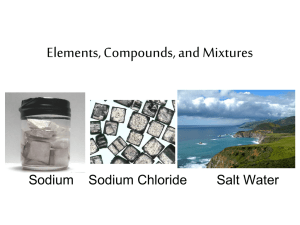
ELEMENTS, COMPOUNDS, AND MIXTURES (Page 66) Please identify the following substances as to whether they are elements, compounds, or mixtures. Remember that an element is made up of only one kind of atom. A compound consists of two or more elements that have been chemically bonded and therefore no longer have the elements’ original properties, but rather brand new properties. A mixture is a combination of two or more kinds of elements or compounds, but NOT chemically bonded. Each ingredient in a mixture keeps its own properties, so that chocolate milk, for example has the properties of chocolate and the properties of milk. Oxygen Gas Carbon Dioxide Gold Air Water Salt Salt Water Vinegar Mercury Helium Nitrous Steel Protein Sugar Iced Tea Baking Soda Lemonade Sulfuric Acid Copper Copper Sulfate A Penny _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ The steps: 1) Can you find the substance on the periodic table? Then it's an element. 2) If the substance is not on the periodic table, ask yourself if you can split it up into smaller parts. 3) If you can't or if the substances are different from the substances it is made of, then it's a compound. 4) If the substance is similar to the substances that it's made of, then it is a mixture. 5) Remember the two tricky cases: a. Metals that are not on the periodic table, like bronze and brass are alloys, which are a kind of mixture. b. When you dissolve one substance into another, that is called a solution, which is another kind of mixture. 6) Also, it helps to ask yourself if you can think of a chemical formula for the substance. If you can, then it is a compound.