Exam 3 Review Sheet Chapter 18 Chem 110b
advertisement

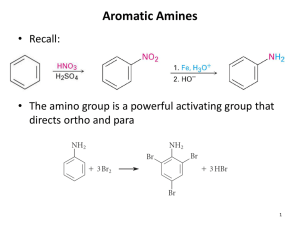
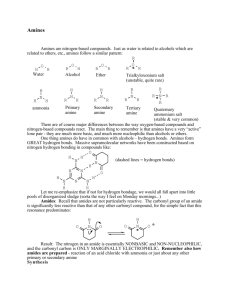
110b Exam 4 Review Sheet- Spring Semester 2010 Review Session: Monday, April 12, 7:00 PM, SN Aud Exam 4: Wednesday, April 14, SN Aud Chapter 20 Goals: 1. Nomenclature: be familiar with the examples on pp. 900-901, including pyridine and sulfanilamide (know a bit of the history behind this drug, see p. 931). Become familiar with the structure and properties of one biologically important amine, p. 910-912. 2. Properties of amines: review lecture notes concerning structural and conformational features. Basicity: you should be prepared to explain trends in basicity among alkyl and arylamines. Know the “ballpark” pKa’s for these two classes of amines (as the protonated forms). 3. Preparation of amines: Gabriel primary amine synthesis, mechanism of reaction/deprotection. 4. Amines via alkylation of amines, reduction of alkyl azides, nitro groups. 5. Reductive amination as a means for 1°, 2°, and 3° amine synthesis. Mechanism of reaction for each case. 6. Reduction of amides, oximes, and nitriles as a means for synthesizing amines. 7. The Hofmann and Curtius rearrangement as methods for amine synthesis. Reaction mechanism. 8. Reactions of amines as nucleophiles. 9. Reactions of secondary alkylamines and primary arylamines with HONO: prep of Nnitrosoamines and aryldiazonium ions. Mechanism of formation, properties of the products. 10. Reactions of aryldiazonium salts: The Sandmeyer reaction (replacement with Cl, H, Br, F, CN, OH) and its utility in direct preparation and/or blocking strategies. Coupling reactions with aryldiazonium salts (dye syntheses) mechanism of reaction. 11. Review the Hinsberg test for structural assignment of 1°, 2°, or 3° amine. 12. Analysis of amines: spectroscopic signatures. 13. Elimination reactions involving ammonium compounds: the Hoffmann and Cope elimination reactions. 14. Be prepared apply any reactions discussed in Chapter 19 (and assigned end of chapter problems) in predict-the-product and synthetic proposals. Chapter 21 1. Nomenclature: be familiar with the examples on pages 955-56. 2. Properties of phenols: be prepared to explain the trends in acidity for substituted phenols. Solubility as an indicator for phenol vs alcohol vs carboxylic acid. 3. Laboratory synthesis (hydrolysis of a diazonium salt) vs industrial syntheses (Dow process, Cumene process) of phenol. Mechanisms of these reactions. 4. EAS reactions of phenols: bromination, nitration, sulfonation, the Kolbe reaction and utility in aspirin synthesis. 5. The mechanism and utility of the Claisen rearrangement. Be familiar with the historical method used to provide evidence for a concerted mechanism. How would you do it today, without resort to radioactive materials? 6. Quinones: synthesis, reactions, and biological utility in redox systems. Allelopathy. 7. NAS reactions via the addition-elimination SNAr mechanism. The Meisenheimer complex. Aromatic substituents that promote the reaction, substituents that act as leaving groups. Thyroxine synthesis. [Learning Group Problem 1, p. 685] 8. NAS reactions via the elimination-addition (benzyne) mechanism. Evidence for this mechanistic intermediate. Roberts’ historical experiment with 14C. Propose an alternative experiment, without resorting to 14C, to provide evidence for the intermediacy of a benzyne intermediate. 9. Spectroscopic signatures of phenols and aryl halides. 10. Work the text problems, be prepared to predict the products, propose syntheses, and address issues outlined above. Special Topic “F”: 1. The DDT story, past and present. 2. The Agent Orange story. 3. The PCB story.