abstract template.
advertisement

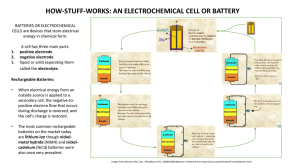
Electrochemical Pathways Towards Sustainability Donald R. Sadoway Department of Materials Science and Engineering Massachusetts Institute of Technology Cambridge, Massachusetts U.S.A. 02139-4307 dsadoway@mit.edu indicate preference: oral presentation or poster [The abstract should be typed, single-spaced, 12 pt Times New Roman font, 2.5 cm margins, page size A4 or US Letter, 250 words maximum]. The importance of electrochemical technologies in sustainable development will be illustrated in three different settings: metals production by molten oxide electrolysis (MOE), which is the electrolytic decomposition of a metal oxide into molten metal and oxygen gas and represents an alternative to today’s carbon-intensive thermochemical metals reduction processes. For MOE the feedstock can be concentrate derived from ore or can be hazardous waste such as chromate sludge; stationary batteries for storage and delivery of off-peak power. Here the emphasis is on colossal current capability, e.g., 100s of kA with a footprint measuring 10s of metres; and portable batteries powering electric vehicles. The enabling material here is a solid, polymer electrolyte with the ionic conductivity of a liquid, the mechanical properties of a solid, and the formability of a commodity thermoplastic. This has been achieved with a class of block polymers that exhibit local segregation, or “micro-phase separation”, into periodically-spaced nanoscopic domains. With such materials it should be possible to construct liquid-free, flexible, thin-film batteries possessing specific energy densities of ~400 Wh/kg at room temperature.