PROBLEM SET-3
advertisement

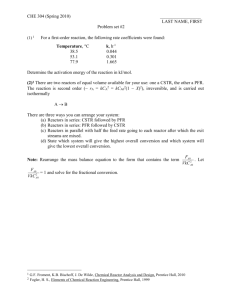
Gazi University Faculty of Engineering and Architecture Department of Chemical Engineering ChE 341 Chemical Reaction Engineering Dr. Çiğdem GÜLDÜR Assist. Dolunay Eslek PROBLEM SET-3 1. Reactant A decomposes with stoichiometry A R and with the rate dependent only on CA. The following data on this aqueous decomposition are obtained in a mixed flow reactor in terms of concentration of A mole/L:CA0=100mole/L, Volumetric flow rate:5L/sec. CA0 200 CA 100 (sec) 14 190 90 25 180 80 29 170 70 30 160 60 29 150 50 27 140 40 24 130 30 19 120 20 15 110 10 12 101 1 20 a)What PFR volume is necessary to achieve 40% conversion? b)What CSTR volume is necessary to achieve 40% conversion? c)What CSTR volume must be added to raise the conversion in part (a) to 90% conversion? d)What PFR volume must be added to raise the conversion in part (a) to 90% conversion? e)Which type of reactor would you prefer to achieve 80% conversion of A in a single reactor or reactor combinations? 2. We are planning to operate flow reactors to convert A into R. This is a liquid reaction, the stoichiometry is A R, and the rate of reaction as a function of concentration is given in table below. CA(mol/L) -rA(mol/L.min) 0.1 0.1 0.2 0.3 0.3 0.5 0.4 0.6 0.5 0.5 0.6 0.25 0.7 0.10 0.8 0.06 1.0 0.05 1.3 2.0 0.045 0.042 a)What size of CSTR is needed for 75% conversion of feed stream of 1000mol A/hr at CA0=1.2mol/L? b) What size of CSTR is needed for 75% conversion of feed stream of 1000mol A/hr at CA0=1.2mol/L? c)Repeat part (a) with the modification that the feed rate is doubled, thus 2000mol A/hr at CA0=1.2mol/L are to be treated . 3. We are planning to operate a batch reactor to convert A into R. This a liquid reaction , the stoichiometry is A R, and the rate of reaction is given in Table. a) What space time is needed for the concentration to drop from CA0 =1.3 mol/L to CAf =0.3mol/L? b) What size of plug flow reactor is needed for 75% conversion of feed stream of 1000 mol A/hr at CA0 =1.2 mol/L. CA(mol/L) -rA(mol/L.min) 0.1 0.1 0.2 0.3 0.3 0.5 0.4 0.6 0.5 0.5 0.6 0.25 0.7 0.10 0.8 0.06 1.0 0.05 1.3 2.0 0.045 0.042 4. An isothermal PFR is used for the gas phase reaction A 2B+C. The feed, flowing at 2.0L/s, contains 50% molA and 50% mol inert species. The rate is first order with respect to A; the rate constant is 2.0s-1. a) Determine the reactor volume required for 80% conversion of A? b) What the volumetric flow rate at the exit of the reactor, if xA=0.8? c) What volume of CSTR would be needed to achieve the same conversion? 5. The irreversible reaction A+B C+D is carried out isothermally in two flow reactors in series, first reactor (V1=10L) is CSTR and second one (V2=40L) is PFR. Inlet concentrations of A and B are 0.01 mol/L and 0.7 mol/L, respectively. The concentration of product C at the exit of the first reactor is 0.002 mol/L. The reaction is first order with respect to B. Find the concentration of C at the exit of the plug flow reactor? 6. The following gas phase reaction takes place in three CSTR is connected in series . A+B C ; -rA= kCACB The volume of the first and second reactors are V1=1L and V2=2L. At the inlet of the first reactor the volumetric flow rate is 0= 0.4 L/s and CA0=CB0=1 mol/L.Reactor operates at 25oC and the reaction rate is 2.6310-3L/mol.s at that temperature. At the exit of the third reactor overall conversion of A is expected to be 0.95. By using graphical method determine the outlet conversions from first and second reactor and the volume of the third reactor. 7. Constant density liquid phase reaction A B+C has the rate law, -rA=k1(CA)1/2/(1+k2CA) k1=10(mol/m3)0.5h-1 k2=16m3/mol FA0=100 mol/hr, CA0=0.25mol/m3 What system (i.e. type and arrangement) of ideal flow reactor(s), either one alone or two in series, would you recommend for continuous processing of a feed pure A if, a) 50% conversion of A is desired b) 90% conversion of A is desired c) What reactor size(s) should be used for part(b)? 8. A first order reaction is to be treated in series of two mixed reactors. Show that the total volume of the reactors is minimum when the reactors are equal in size. 9. A liquid phase reaction A+2B C is to be conducted into two equal –sized CSTRs. The reaction is first order with respect to both A and B with kA=6.010--2Lmol-1s-1. The volumetric feed rate is 25 L.min-1, CA0=0.1 molL-1 and CB0=0.2 molL-1; 75% conversion of A desired. a) The CSTRs are arranged in series . What is the volume of each? b) The CSTRs are arranged in parallel. What is the volume of each, if the volumetric flow rate through each reactor is equal? 10. The liquid phase reaction A B+C takes place in series of three CSTRs with an overall conversion (xA) of 0.90. One of the reactors developes a leak and is removed from the series. The throughput (V) is then reduced in order to keep the overall conversion at 0.90. ıf the original throughput was 250Lmin-1, what is the new thrughput? Assume: (i) steady-state operation;(ii) the reactors are of equal volume and operate at the same temperature ;(iii) the reaction is first order in A. 11. Determine the reactor configuration and the minimum total volume for the reaction A+B products with the rate law -rA= k1CA/(K2+CA+CB) The desired fractinal conversion is 0.85. Data: k1= 0.25 molL-1min-1 CA0=CB0=0.25 mol/L-1 K2=0.50 molL-1 0=5.0 Lmin-1 12. Problems 6.1, 6.3, 6.5, 6.7 The textbook: Levenspiel O., “Chemical Reaction Engineering”,3rd ed.(1999). Solve problem 11 as your homework on 8th of November, 2008.