Mixture - A physical combination of two or more substances that are
advertisement

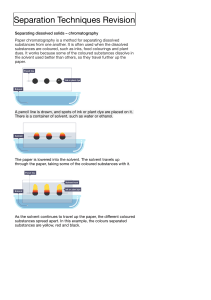
Mixture - A physical combination of two or more substances that are blended together without forming new substances. The combined substances retain their individual properties and can usually be separated fairly easily. Solution - A mixture of substances that are blended so completely that the mixture looks the same everywhere. Solute - A substance that is dissolved by another substance to form a solution. Solvent - A substance that dissolves one or more other substances to form a solution. Saturation – Dissolving a substance in a solvent until the solvent can absorb no more. For example, we did this when we dissolved salt to create salt crystals during our rocks and minerals study. Chemical change - A change of matter that occurs when atoms link together in a new way, creating a new substance different from the original substances. Generally speaking, you CANNOT reverse a chemical change. Examples of chemical change include, cooking, tarnishing, rotting, burning, rusting, and digesting. Nothing is ever lost and nothing is ever gained in a chemical reaction. Exothermic – A chemical reaction that gives off heat. Indications of an exothermic reaction would include seeing fire, flames, feeling heat, or even a slight temperature increase. Endothermic – A chemical reaction that absorbs energy/heat. Indications of an endothermic reaction could be feeling cool or even cold. Conservation of Matter – a basic law of science that says that matter can never be created or destroyed, only changed in form. Acid – A substance that tastes sour and turns blue litmus paper red. Base – A substance that tastes bitter and turns red litmus paper blue. Neutral – A substance that is neither an acid or a base. An example is water. Acidity – The strength of an acid. Alkalinity – The strength of a base.