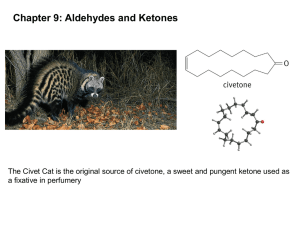
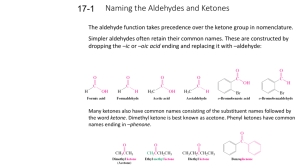
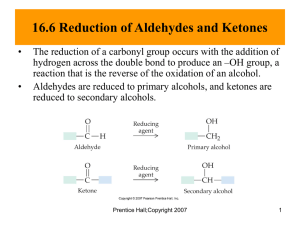
Chapter 17: Carbonyl Compounds II
advertisement

Chapter 17: Carbonyl Compounds II HW: 45, 46, 47, 48, 49, 50, 52, 53, 55, 56, 58, 59, 60, 63, 64, 65, 66, 67, 71, 83 I. Nomenclature A. Aldehydes B. Ketones C. Functional Group Hierarchy (Table 17.1 p791) 1. list by parent/root (suffix) and substituent (prefix) name II. Nucleophilic Attack on Carbonyls Revisited A. Relative reactivities acyl halide > anhydride > aldehyde > ketone > ester ~ carbox acid > amide > carbox salt B. General reaction 1. Carbonyl Compounds I with leaving group 2. Carbonyl Compounds II with no leaving group (aldehydes/ketones) a. strong base/nucleophile (1st step irreversible) b. weak base/nucleophile (fully reversible) 1 c. Grignard (lengthening the C chain) i. aldehyde to 2º alcohol ii. ketone to 3º alcohol iii. formaldehyde to carboxylic acid iv. mechanism d. Reactions with Acetylide ions (lengthening the C chain II) e. Reactions with a hydride ion (1. NaBH4 i. aldehyde to 1º alcohol 2. H+) ii. ketone to 2º alcohol iii. acyl halide to 1º alcohol 2 iv. mechanism v. or ester or carboxylic acid to 1º alcohol (using LiAlH4) vi. esters into aldehydes using DIBAL(diisobutylaluminum hydride) at -78º C and follow with water (p802) (strange since aldehydes more reactive than esters) vii. amide to amine f. Reactions with cyanide (lengthening the C chain III) i. works in acid, not base soln ii. further reactions w/cyano (H2/Pt) or (HCl/heat/H2O) g. Reactions with amines and derivatives (enamine/imine p807) 3 III. More nucleophilic attacks on aldehydes and ketones A. Water (gem-diols or hydrates) (generally unstable) 1. mechanism (acid catalyzed) B. Alcohol 1. hemiacetal and acetal (I sometimes call it “full” acetal just to differentiate it from hemiacetal, but that’s not “official”) 2. hemiketal and ketal (again, “full” ketal sometimes) 3. hemis not usually stable except as cyclics IV. Protecting Groups A. Using a cyclic ketal/acetal to protect ketone/aldehyde (p819) 4 B. Using thioketals/thioacetals (p822) 1. removal with Raney Ni produces alkane V. Wittig Reaction (p822) A. Lengthens the chain with an alkene where carbonyl was VI. Stereochemistry A. Prochiral (Re and Si) VII. Retrosynthesis VIII. 1,4 addition on A. ketones/aldehydes 5 B. Other carbonyls Objectives Knowledge Recall IUPAC nomenclature for aldehydes and common names for one and two carbon aldehydes (including designation) Recall IUPAC and “derived” nomenclature for ketones Memorize where aldehydes and ketones are in relative reactivity to nucleophiles (p736) Define the terms carbonyl, carbon, cyano, nitrile, nucleophillic addition, nucl. addn.elimination, cyanohydrin, imine, enamine, oxime, hydrazone, semicarbazone, hydrate/gem-diol, acetal, ketal, hemiacetal, hemiketal, protecting grps, ylide, Wittig rxn, Re and Si faces List the priorities for functional groups (p791) Recall from previous chapters: acetylide ion, nucleophillic acyl substitution Comprehension Understand why aldehydes are more reactive than ketones Explain why ester’s or acyl halide reaction with a Grignard is different from ketone Interprete a pH-rate profile plot (in this case, for imine formation) Application Use reactions in summary (836-840) to modify molecules Write a mechanism for a nucleophillic addn. or nucl. addn. elimination reaction Employ , unsaturated carbonyl compounds as conjugate addn rxns Analysis Use retrosynthesis tools in this chapter to figure out steps to get desired products Synthesis Use known reactions to create other target molecules 6