atmosphere outline - Santa Monica College
advertisement

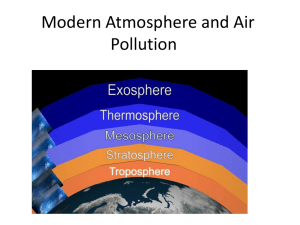
Vicki Drake Santa Monica College Earth Sciences Department ATMOSPHERE OUTLINE I. Origin of Earth’s Atmosphere A. Original atmosphere: Helium and Hydrogen B. Outgassing from volcanoes: H2O (water vapor) 1. Condensation and precipitation to form world’s oceans C. CO2 dissolved from oceans and locked into rocks (limestone) D. Nitrogen (an opportunistic little gas) moved in with Helium and Hydrogen E. Oxygen initially released by photodissociation of H2O F. Oxygen also produced by Blue-Green Algae – Stromatolites 1. 3.5 billion years ago II Composition of Atmosphere Permanent Gases A. Nitrogen - ~78% B. Oxygen - ~21% C. Remaining ~1% 1. Argon 2. Neon 3. Helium 4. Hydrogen 5. Xenon Variable Gases A. Water Vapor (H2Ov) B. Carbon Dioxide (CO2) C. Methane (CH4) D. Nitrous Oxide (N2O) E. Ozone (O3) F. CFCs (Chlorofluorocarbons) G. Particulates (Microscopic soot, ash, dust, salt crystals, etc.) III Greenhouse Gases: trap long-wave Infrared (heat) radiation from Earth A. Carbon Dioxide 1. Held by plants during photosynthesis 2. Held in world’s ocean by phytoplankton 2. Released through burning of fossil fuels a. Natural gas b. Coal c. Oil d. Released through deforestation processes © Vicki Drake Santa Monica College Spring 2002 Lectures B. Methane 1. Formed during decay process of organic material 2. Released through Rice paddies (shallow, flooded fields) 3. Released through biochemical reactions in stomachs of cattle 4 Released through anaerobic decay in landfills 5 .A more powerful greenhouse gas than CO2 6. Can be removed from atmosphere by hydroxyl radicals C. Nitrous Oxide 1. Formed by chemical processes between bacteria, microbes and soils 2. Released through “tilling” of soils for agriculture 3. Broken down through photodissociation by Solar UV rays. 4. Nitrogen released plays role in development of photochemical smog. D. Ozone 1 Formed through photochemical reactions of O and O2 and Solar Energy 2. Lower atmosphere: irritant to mucous membranes, eyes, lungs, etc. 3. Upper atmosphere: absorbs UVA and UVB solar energy 4. Formed in Stratosphere ~ 30 km above Earth’s surface a. A Protective layer slowly being eradicated by anthropological processes E. CFCs (Chlorofluorocarbons) 1. A manmade compound used for: a. Insulation b. Refrigeration c. Propellant 2.Stable in lower atmosphere a. Inert, non-reactive b. Non-carcinogenic 3.Unstable in upper atmosphere a. Breaks down into components of 1. Chlorine 2. Fluorine 3. Carbon 4. Chlorine 1. Reactive, unstable 2. Seeks out and destroys Ozone F. Particulates a. Microscopic (~10 diameter) bits of soot, ash, etc IV. Introduction to Pressure A. Most of atmosphere is compressed at Earth’s surface by Gravity B. Air is matter having properties of weight (due to gravity) and mass (due to quantity of molecular material) C. The Density of air is a ratio of the mass of the air the the amount of space between each molecule (D=mass/volume) © Vicki Drake Santa Monica College Spring 2002 Lectures D. Air molecules push against each other with a specific force. The push can be measured as Pressure (Pressure = force/area) E. At sea level, average air pressure = 14.7 lbs/in2. F. A more common way of expressing pressure is in millibars (sea level air pressure 1013.25 mb) 1. A bar is a unit of pressure (force of 100,000 Newtons on a surface area of 1 meter2) 2. 1000 millibars = 1 bar G. Higher into the atmosphere – less air molecules available. a. Density of Air decreases with increasing altitude H. Lower density of air = lower Air pressure a. Air pressure decreases with increasing altitude V. Layers of Atmosphere A. Lapse Rate a. Changing air temperature with a changing altitude b. Normal Lapse Rate i. Air temperature decreases with an increase in altitude c. Inverse Lapse Rate i. Air temperature increases with an increase in altitude d. Isothermal Lapse Rate i. No change in air temperature with an increase in altitude e. Environmental Lapse Rate: ~ 6.50C/1000 meters (3.60F/1000 feet) B. Troposphere a. Lowest layer of the atmosphere b. Elevation from the surface to ~ 11 km above the surface i. Average height greatest at equator, least at poles c. Well-mixed layer with rising and falling air d. Normal Lapse Rate in effect e. All “weather” occurs here – storms, winds, etc. C. Tropopause a. Top of troposphere b. Isothermal lapse rate c. Altitude varies, but ~ 11km – 20km above the surface D. Stratosphere a. Second layer of atmosphere b. Elevation from ~20km – 55 km above surface c. Most of upper atmosphere Ozone formed here (greatest quantity @30km) d. Inverse Lapse rate in effect due to Ozone absorbing Solar energy E. Stratopause a. Top of stratosphere b. Isothermal lapse rate c. Altitude varies but ~55 km to 60 km above surface F. Mesosphere a. Third layer of atmosphere b. Elevation from ~ 60 km to 80 km above surface c. Few air molecules, atmospheric pressure <1 millibar © Vicki Drake Santa Monica College Spring 2002 Lectures d. Percentage of Nitrogen to Oxygen still same as lower atmosphere (just fewer molecules) e. Normal lapse rate – little Ozone or air molecules available G. Mesopause a. Top of mesosphere b. Isothermal lapse rate c. Altitude varies but ~ 80-85 km above surface H. Thermosphere a. Fourth layer of atmosphere b. Elevation from ~ 85 km to 500 (?) km above the surface c. Inverse lapse rate – mainly due to O2 absorbing solar energy and breaking into O + O atoms. I. Exosphere a. “Top” of the atmosphere b. Boundary between Earth’s atmosphere and “outer space” c. Variable boundary ~500 (?) km to ? km H. Ionosphere a. Not a true separate layer, but rather an electrified region of upper atmosphere (ranging from ~60 km to 500 km b. Large concentrations of ions and free electrons exist here c. Radio communications use ionosphere © Vicki Drake Santa Monica College Spring 2002 Lectures