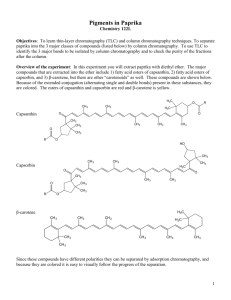
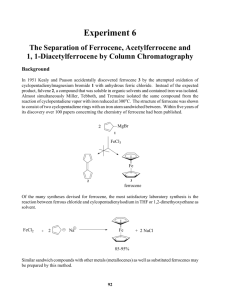
column chromatography
advertisement

CHEM2411L Clayton State University Dr. Susan F. Hornbuckle Experiment #4 Part 2 - Column Chromatography ISOLATION OF CAFFEINE Place two tea bags in a 125-ml Erlenmeyer flask with 50 ml of distilled water and bring the mixture to a gentle boil. After 2 minutes, remove the heat and allow the solution to cool. Squeeze out the tea bags and discard them. Dilute the aqueous tea solution with 20 ml of 5% aqueous HCl to help prevent emulsion formation, and extract the resulting solution with four 10ml portions of methylene chloride. Do not shake the separatory funnel vigorously; instead, swirl it with moderate vigor so an emulsion might be avoided. The combined methylene chloride extracts are dried over Na2SO4 and decanted into a clean dry 100mL round bottom flask. Add silica gel (0.2g) to the round bottom flask and evaporate the solvent on a steam bath or rotary evaporator to give a dry powder. The chromatographic column is packed in the following manner: Use 10 x 150-mm column and 0.7g of silica gel. 1) Push a small wad of glass wool into the bottom of the column. 2) Gently pour a layer of sand over the glass wool and tap the column to settle the sand. The sand retains fine particles and also provides a flat horizontal base for the adsorbent column. 3) Add weighed amount of dried silica in a fine stream and gently tap tube to dislodge air bubbles and to level the top of the silica gel in the column. 4) Add your sample from the round bottom flask to the top of the silica gel in your column, and tap to settle this new layer. 5) Add a layer of sand (both the top and bottom sand layers should be about 0.5cm thick) to prevent disturbance of the surface when solvent is added. Tap again to settle new layer. Eluting a column: i) Starting adding the first solvent (three 5-ml portions of methylene chloride solvent followed by two 5-ml portions of pure ethyl acetate) very gently, so as not to disturb the sand on top of the column. When the solvent starts to drip out if the bottom of the column, begin collecting fractions- collect approximately 5 ml in each numbered (#1 #5) test tube. Keep adding solvent to the top of the column so it does not go dry. If the column runs slowly, gentle air pressure may be applied. Note: Be sure to have your solvents and collecting test tubes ready before applying the solvent to column. ii) The contents of each test tube may be checked by TLC with comparison to an authentic sample of caffeine using silica plates and 1-butanol/ethyl acetate/methyl isobutyl betone (2:9:9) as the solvent. Spot each tube of eluate several times in one place to make sure that enough sample is applied. If the column has worked properly, early yellow fractions that smell like tea will be followed by fractions that contain caffeine. Combine the caffeine containing fractions in a clean dry tared round bottom flask and evaporate solvent on the rotary evaporator. Obtain the mass of your isolated caffeine sample and a melting point.