Exam 2 Review
advertisement

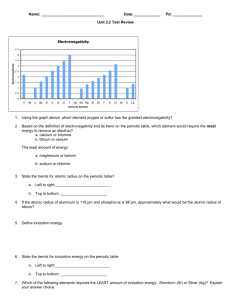
Exam 2 Review Supplemental Instruction Iowa State University Leader: Course: Instructor: Date: Emily M Chem 167 Houk 3/10/14 1. Of the following, which gives the correct order for atomic radius for Mg, Na, P, Si and Ar? a) Mg>Na>P>Si>Ar b) Ar>Si>P>Na>Mg c) Si>P>Ar>Na>Mg d) Na>Mg>Si>P>Ar e) Ar>P>Si>Mg>Na 2. Which of the following species has the larges radius? a) Rb + b) Sr 2+ c) Br – d) Kr e) Ar 3. [Ar]4s23d104p3 is the electron configuration of a(n)___________ atom. 4. What is the ground state electron configuration for Si? 5. Which of the following is an excited-state electron configuration? a) 1s2 2s2 3p1 b) 1s2 2s2 2p6 c) 1s2 2s2 2p4 3s1 d) [Ar] 4s2 3d10 4p1 6. Which of the following has the smallest ionization energy. a) Mg b) Se c) Ba d) Po 7. Which one of the following sets of quantum numbers corresponds to a 6p electron? a) n= 6, l= 1, ml= -2, ms= +1/2 b) n= 6, l= 2, ml= 0, ms= -1/2 c) n= 5, l= 1, ml= -1, ms= -1/2 d) n= 6, l= 1, ml= -1, ms= +1/2 1060 Hixson-Lied Student Success Center 515-294-6624 sistaff@iastate.edu http://www.si.iastate.edu 8. Which one of the following represents an acceptable possible set of quantum numbers (in the order n, l, ml, ms) for an electron in an atom? a) b) c) d) e) 2, 1, -1, +1/2 2, 1, 0, 0 2, 2, 0, +1/2 2, 0, 1, -1/2 2, 0, 2, +1/2 9. The wavelength of a photon that has energy of 6.33*10-18J is _______m 10. The retina of a human eye can detect light when radiant energy incident on it is at least 4.0 x 10-17 J. For light of 600 nm wavelength, how many photons does this correspond to? 11. What is the frequency of yellow light with wavelength of 595 nm? 12. Consider the sine waves representing light or electromagnetic radiation. Which one corresponds to photons with the largest energy? 13. The energy required to remove an electron from metal X is ΔE = 3.31 x 10-20 J. Calculate the maximum wavelength of light that can photo eject an electron from metal X. 14. A neon atom emits light at many wavelengths, two of which are at 616.4 and 638.3 nm. Both of these transitions are to the same final state. a) What is the energy difference between the two states for each transition? b) If a transition between the two higher energy states could be observed, what would be the frequency of the light? 15. Given the electronegativities below, which covalent bond is most polar? Element: Electronegativity: a. b. c. d. e. H 2.1 C 2.5 N 3.0 O 3.5 C-H N-H O-H O-C O-N 16. Among the following molecules, only _______ is polar. a. BeCl2 b. CBr4 c. NF3 d. AlCl3 e. Cl2 17. Draw Lewis structures for the following molecules. Give the shape of the molecule, hybridization of the central atom, and the bond angle(s). a. CH2F2 b. OF2 c. PO3 3- d. BCl3 e. BeF2 f. PBr5 18. The total number of σ bonds in the H-CΞC-CΞC-CΞC-CΞN molecule is a) 4 b) 6 c) 8 d) 12 e) 15 19. In ethylene (H2C=CH2), the C=C double bond results from _____ overlap of _____ orbitals and ______ overlap of ______ orbitals on the C atoms. a) σ, sp, π, p b) σ, sp2, π, sp2 c) σ, sp3, π, s d) σ, sp2, π, p e) π, sp2, σ, p 20. Which drawing represents a π bonding molecular orbital for a homonuclear diatomic molecule? 21. Which of the following molecular species is stabilized by resonance? a) CO2 b) N3c) BH3 d) O22e) HCN 22. What is the coordination number for (a) simple cubic (b) body-centered cubic centered cubic? (c) face- 23. Nickel crystallizes in a face-centered cubic lattice. If the density of the metal is 8.908 g/cm3, what is the unit cell edge length in cm? 24. How does the “sea of electrons” model of metallic bonding explain why metals are good conductors of electricity? 25. __ - type doping involves a dopant with more valence electrons than the substance. __ type involves a dopant with fewer valence electrons. 26. Rank the following elements in order of increasing conductivity by considering the band theory of bonding in solids. (a)Sr (b)Ti (c)C (d)Si doped with As (e)Si