Physical Sciences Lesson Plans for week of: K
advertisement

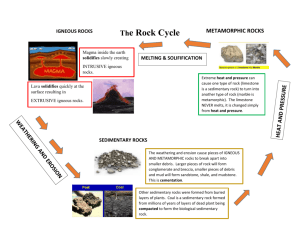
Physical Sciences Lesson Plans for week of: Sr. Physics Mon. October 3rd hr. Jr. Chem 19 2015 K. Kinzer 1st hr. Fr. PreChem/Phys 4th, 5th, hrs. 4th 5th 1. Students will discover conservation 1. Students will review gas stoichiometry problems of velocity by a “colliding marbles” 2. Define temperature in terms of lab a. Ek vs. temp overhead demo 2. Review test b. historical molecular speed exp. Hand out Ch 3,4 ws 1. Students will complete Mystery Powder lab by a. ID 2nd unknown powder b. discuss lab reports 2. Begin atomic theory a. basic questions to answer b. atom & history (Ch 4 2nd ½ hw is due) Tue. 1. Students will review conservation of 1. Students will define the S L phase change by melting a velocity by review of lab vectors compound - Exp 13 2. Define Newtonian philosophy as whole equals sum of parts Hand out momentum lab 1. Students will define direct vs. indirect evidence & apply to “black box” exercise 2. Begin atomic structure/demo by magnetism of matter 3. Begin electricity of matter (Mystery powder lab reports due) Wed. 1. Students will apply conservation of 1. Students will review temp vs. particles Ek vs. speed vs. mass momentum to a lab on “colliding 2. Review gases & stoichiometry by marbles” of different masses a. PhET simulation b. Ch 4 hw review c. board problems 1. Students will review 2 types of evidence by homework review 2. Complete statics demo & evidence for atomic structure 3. Apply demo results to model of atom (velocity lab report is due) Thur. 1. Students will continue Newtonian view of nature 2. Define momentum in terms of a. m x v b. vectors of momentum lab c. apply to simple problems 3. Begin 1-D collisions (Reader Reports are due) Fri. 1. Students will continue momentum by ESPN video “Running with momentum” 2. Apply 1-D collisions to problems 3. Define open vs. closed systems (momentum lab report is due) 1. Students will consolidate information 1. Students will define the electron, on stoichiometry and gases by a test proton, and evidence for neutron 2. Discuss ChemMatters magazine articles Hand out lesson 12 sheet (corrected Ch 3,4 ws is due) (lost part of hour) 1. Students will review test 1. Students will summarize atomic 2. Define solid vs. liquid vs. gas by structure basics by quiz completion a. physical properties 2. Discuss ChemMatters article reports b. intermolecular forces 3. Apply periodic table to determining 3. Review Exp 13 time vs. temp graphs the structure of different atoms How It Works ? 6th hr. 8th Earth Science Mon. 1. Students will apply igneous rocks to internet search on the “Boulder Batholith” and local geology (Igneous lab & internet work is due) Tue. 7th hr. 1. Students will review test 2. Define elements as a. what they are b. parts of periodic table c. get symbols to memorize top 12 (notebooks due) (turn in water lab reports) 1. Students will discuss significance of 1. Students will rev. element symbols 2. Define compounds in terms of the Boulder Batholith a. what they are 2. Review igneous rocks 3. Define sedimentary rocks in terms of b. chemical formulas 3. Define mixtures vs. solutions a. general information b. organic vs. inorganic c. texture - particle size Hand out Sedimentary rock lab Wed. (text reading notes due) 1. Students will identify sedimentary 1. Students will apply mixtures & rock samples by work on a properties solutions to 3 types of water 2. Continue traits of water by testing lab their hydrometers IET 6-1 3. Begin salt water as a solution Thur. 1. Students will discuss home rock labs 1. Students will continue salt water a. solutions 2. complete sedimentary rocks by b. % and meanings a. clastic vs. chemical formation c. go over water lab report b. review sedimentary lab work 2. Define matter in terms of 3. Begin metamorphic rocks a. 4 phases b. 4 classes 3. Define kinetic vs. potential energy (Sedimentary rock labs due) Fri. 1. Students will review sedimentary 2. Define metamorphic by a. basic formation b. 2 major groups Hand out Metamorphic Rock lab 1. Students will apply % salinity to H2O density by work on IET 6-2 lab 2. Begin polarity of water in terms of a. sticky (+) vs. (-) b. adhesion vs. cohesion Hand out matter & water ws