Review: Atoms Name: ____Mr. John_____________ Date
advertisement

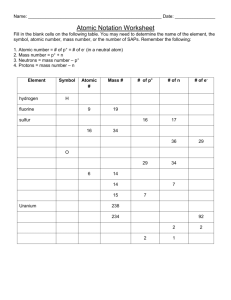
Review: Atoms Name: ____Mr. John_____________ Date: ____KEY______________ 1. The 3 particles of the atom are: a. proton b. neutron c. electron 2. Their respective charges are: a. positive b. neutral c. negative 3. The number of protons in one atom of an element determines the atom’s identity, and the number of electrons determines its electrical charge. 4. The atomic number tells you the number of protons in one atom of an element. It also tells you the number of electrons in a neutral atom of that element. The atomic number gives the “identity “of an element as well as its location on the Periodic Table. No two different elements will have the same atomic number. 5. The atomic mass of an element is the average mass of an element’s naturally occurring atoms, or isotopes, taking into account the percentage of each isotope. 6. The atomic mass of an element is the total number of protons and neutrons in the nucleus of the atom. 7. The mass number is used to calculate the number of neutrons & electrons in one atom of an element. In order to calculate the number of neutrons you must subtract the atomic number from the atomic mass. 8. Give the symbol and number of protons in one atom of: Lithium Li, 3____________ Bromine Br, 35___________ Iron Fe, 26________________ Copper Cu, 29___________ Oxygen O, 8_____________ Mercury Hg, 80____________ Krypton Kr, 36__________ Helium He, 2_____________ 9. Give the symbol and number of electrons in a neutral atom of: Uranium U, 92______________ Chlorine Cl, 17________________ Boron B, 5_______________ Iodine I, 53_________________ Antimony Sb, 51______________ Xenon Xe, 54____________ 10. Give the symbol and number of neutrons in one atom of: (To get “mass number”, you must round the “atomic mass” to the nearest whole number) Barium Ba, 81______________ Bismuth Bi, 126_____________ Carbon C, 6________________ Hydrogen H, 0_____________ Fluorine F, 10_______________ Magnesium Mg, 12__________ Europium Eu, 90___________ Mercury Hg, 121____________ 11. Name the element which has the following numbers of particles: a. 26 electrons, 29 neutrons, 26 protons Iron, Fe________ b. 53 protons, 74 neutrons Iodine, I__________________ c. 2 electrons (neutral atoms) Helium, He_____________ d. 20 protons Calcium, Ca_____________ e. 0 neutrons Hydrogen, H_____________ 12. At a minimum, what information must you have to determine the identity of an element? # of P+ A A typical isotopic symbol takes this form: Z X Where: X = element symbol; A = mass number [# of protons (p) + # neutrons (n)]; . Z = atomic number [# of protons]; N = # of neutrons; A – Z = N 13. Fill in the missing items in the table below. isotopic symbol Name symbol Z A #p #e #n sodium Na 11 23 11 11 12 23 11 Na chlorine Cl 17 35 17 17 18 35 17 Cl potassium K 19 39 19 19 20 39 19 K symbol Z A #p #e #n phosphorus P 15 31 15 15 16 iron Fe 26 56 26 26 30 56 26 iodine I 53 127 53 53 74 127 53 Name symbol Z A #p #e #n isotopic symbol silver Ag 47 108 47 47 61 108 47 Ag krypton Kr 36 84 36 36 48 84 36 Kr tungsten W 74 184 74 74 110 184 74 W Name isotopic symbol 31 15 P Fe I 14. In a neutral atom, # Protons = # Electrons: True or False? 15. Thallium has two isotopes: thallium-203 and thallium-205. Thallium's atomic number is 81, and its atomic mass is 204.38 amu. Which statement about the thallium isotopes is true? a. There is more thallium-203 in nature c. Thallium-205 atoms have fewer neutrons b. Atoms of both isotopes have 81 protons d. The mass of the most common Tl atom = 204.38 amu. 16. Atoms consist of: (check all that apply) Protons Neutrons Molecules Electrons 17. Which of these descriptions is incorrect? Proton: positive charge, in nucleus, mass of = 1 amu Electron: negative charge, mass of = 0 amu, in nucleus Neutron: mass of = 1 amu, no charge 18. The atom that has atomic mass of 12 has ___6____________ protons. 19. What do you call an atom that is positively or negatively charged because it gained or lost one or more electrons? Ion 20. The valence electron(s) is/are the outer most electron(s) in an atom. 21. The center of an atom is called the: nucleus 22. In an atom, the electrons form a cloud around the nucleus. 23. Objects that are electrically neutral become negatively charged when they gain electrons. 24. In an element with a neutral charge, the atomic number is equal to the number of p+ & e‒. 25. _____________ are particles with no charge. Protons Neutrons Electrons 26. _____________ are negatively charged particles. Protons Neutrons Electrons 27. _____________ are positively charged particles. Protons Neutrons Electrons 28. The atom has the following parts ions, protons, electrons protons, electrons, neutrons protons, electrons, molecules elements, neutron protons 29. What charge do protons carry? Positive 30. Is an atom the smallest particle of matter? True Negative Neutral False 31. Determine the average atomic mass of this mixture of naturally occurring isotopes of Barium. Isotope N.A.% amu Ba - 130 0.106% 0.1378 Ba - 132 0.101% 0.13332 Ba - 134 2.417% 3.23878 Ba - 135 6.592% 8.8992 Ba - 136 7.854% 10.68144 Ba - 137 11.23% 15.3851 Ba - 138 71.7% 98.946 Sum = 137.4216 32. Some possible sources of experimental error are: Inaccurate measurements; flawed methodology; poor experimental design; careless practices; inadequate or inferior tools; unforeseen circumstances or occurrences.