Preparing Solutions
advertisement

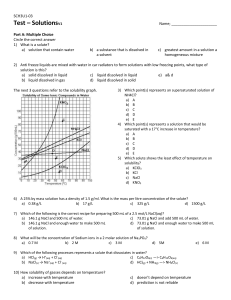
Preparing Solutions Solutions are made when a solute dissolves in a solvent. The most commonly used solutions in high school labs involve a solid solute (usually powdered) dissolved in water or another liquid. Solutions are identified not only by their ingredients, but also by the relative strength of each component. The relative amount of solute to solvent is measured by a unit called a mole (M). A 1 molar solution is a solution in which 1 mole of a compound is dissolved in a total volume of 1 liter. IMPORTANT NOTE: This does NOT mean that 1 mole of a compound is dissolved in 1 liter of solvent. The total volume of the solution should equal 1 liter. To measure 1M of a substance, calculate the molecular weight of the substance and convert to grams. Example: Sodium Chloride (NaCl) has a molecular weight of 58.44 (one Na at 22.99 plus one Cl at 35.45 equals 58.44). So 58.44 g of NaCl is used for 1L of a 1M solution of NaCl. To make 1L of a 1M solution of NaCl: Combine 58.44 g NaCl with about 700 mL water. Stir until dissolved. Pour the solution into a 1000 mL graduated cylinder. Add water to bring the volume up to 1000 mL (1L). To make a less concentrated solution: Decrease the amount of solute proportionally (5.8 g for 100 mL) OR increase the amount of solvent proportionally OR prepare the 1M solution and then dilute. For .4M solution, combine 60 mL of the 1M solution with 90 mL water. Complete the following: Solute Calculation Salt (NaCl) (1 * 22.99) + (1 * 35.45) Calcium Carbonate (CaCO3) (1 * _____ ) + (1 * _____ ) + (3 * ______) Sucrose (C12H22O11) Glucose (C6H12O6) Molecular Weight grams for 1L of 1M solution Preparing Solutions LAB Prepare ½ L of a 1M solution of sucrose. Calculate grams of sugar for 1L and divide by 2: ________________ g Weigh sugar (add weight of container) Add measured sugar to approximately 300 mL water; mix thoroughly. Pour into graduated cylinder and bring water level up to 500 mL. Prepare 150 mL of each diluted solution: .8M, .6M, .4M, .2M Calculate proportions of each dilution (Find quantity needed to make 150 mL.) Concentration 1M sucrose solution distilled water 1M .8M .6M .4M .2M Label your containers with each concentration level. Measure the correct amount of the 1M solution in a graduated cylinder. Add water to bring to 150 mL. Pour into the labeled container. Retain 150 mL of the 1M solution in a labeled container.