pH Math Practice (Chapter 3) ANSWERS Complete the following
advertisement

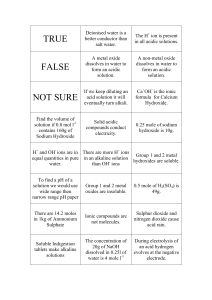
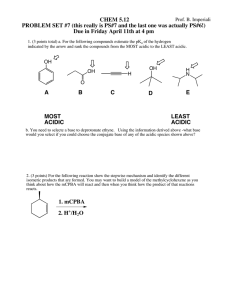
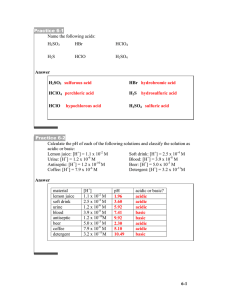
pH Math Practice (Chapter 3) ANSWERS 1) Complete the following table to review your understanding of pH [H+] [OH-] pH Acidic, Basic, or Neutral? 10-3 10-11 3 Acidic 10-8 10-6 8 Basic 10-7 10-7 7 Neutral 10-1 10-13 1 Acidic 2) The carbonic acid/ bicarbonate system is an important biological buffer. Label the molecules and ions in this equation and indicate which is the H+ donor and acceptor. H2CO3 ↔ HCO3- + H+ H+ donor H+ acceptor (acid) (base) 3) In which direction will this reaction proceed a. b. when the pH of a solution begins to fall? To the left when the pH rises above normal level? To the right 4) HCl is a strong acid that dissociates in water: HCl H+ + Cl- What is the pH of 0.01M HCl? [H+] = 10-2 M pH = 2 5) Compared with a basic solution at pH 9, the same volume of an acidic solution at pH 4 has 105 (or 100,000) times as many hydrogen ions (H+). 6) Given a liter of pure water and a liter solution of carbonic acid, what would happen to the pH if you added 0.01 mol of a strong acid to each? Adding strong acid to water would make the solution much more acidic. Adding strong acid to carbonic acid (A BUFFER!) would only make the solution slightly more acidic. 7) Suppose the hydroxide ion concentration in vinegar is 1x10-11 M. Calculate the pH. [OH-] = 10-11 M [H+] = 10-3 M pH = 3 8) Acid precipitation has lowered the pH of a particular lake to 4.0. What is the hydrogen ion concentration of the lake? What is the hydroxide concentration of the lake? [H+] = 10-4 M [OH-] = 10-10 M