Learning Outcomes Sheet for test # 13 Name : Unit 2, Lessons 1
advertisement

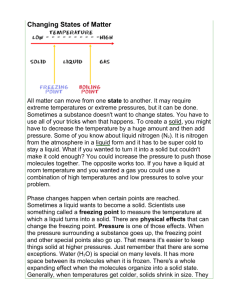
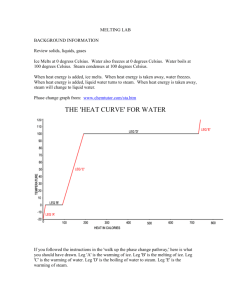
Learning Outcomes Sheet for test # 13 Unit 2, Lessons 1-2 Name : ________________________________ Vocabulary: Students need to learn and understand the meaning of the following vocabulary words AND be able to apply them to a variety of situations. 1. physical change – a change of matter that does not change the type of matter 2. heat- the flow of energy between objects with different temperatures; it can cause water and other matter to change states, or phases 3. evaporation – the change of a liquid into a gas below the boiling point 4. condensation – changing of a gas to a liquid 5. sublimation – the process of changing directly from a solid into a gas without first becoming a liquid 6. melting point – the temperature at which a substance melts; melting point of water is 0 degrees Celsius/32 degrees Fahrenheit 7. boiling point – the temperature at which a substance boils; boiling point of water is 100 degrees Celsius/212 degrees Fahrenheit 8. freezing point – the temperature at which a substance freezes; freezing point of water is 0 degrees Celsius/32 degrees Fahrenheit 9. compound – a substance that is formed by the chemical combination of two or more elements and that acts like a single substance 10. chemical reaction – a change of matter that occurs when atoms link together in a new way, creating a new substance 11. reactant – an original substance at the beginning of a chemical reaction 12. product – a new substance produced by a chemical reaction 13. precipitate – a solid formed from the chemical reaction of some solutions 14. law of conservation of mass – the total number of each type of atom must be the same in the reactants and the products Students will learn in class about the following topics. Be prepared to be assessed on them. 1. A physical change alters the form of an object without changing what type of matter it is. Examples of physical changes: sharpening a pencil, changing states of matter (like solid to liquid, or liquid to gas, etc.), evaporation, condensation, sublimation, melting, boiling, freezing; cutting paper 2. What makes any material change from one state to another? A change in heat energy 3. What must be done to a solid to change it into a liquid? Heat must be added 4. Does a gas gain heat when it condenses? No, it loses heat because the particles move more slowly 5. What happens to the particles of a substance as heat is added? Heat energy causes the particles to move faster and farther apart. 6. Dry ice can sublimate at room temperature. Water can also sublimate. The frost in a freezer comes from gaseous water sublimating out of uncovered food or ice cubes. 7. How does ice keep drinks cool? It keeps drinks cool mainly by melting. Ice absorbs heat from the liquid drink. This heat changes the state of the ice instead of raising its temperature. 8. Why is the melting point of water the same temperature as the freezing point of water? The process of melting and freezing both describe what happens between the solid and liquid states of matter. 9. If something has a very high melting point or boiling point, it means that it takes a lot of heat energy to change the motion of the particles in the substance. 10. BE ABLE TO TELL HOW MANY ATOMS THERE ARE OF A SUBSTANCE IN A MOLECULE. For example: NaHCO3 How many Oxygen atoms are in this molecule? 3 11. Explain the chemical reaction that takes place in photosynthesis. Use the chemical equation to help you. 6CO2 + 6H2O ----> 6O2 + C6H12O6 The reactants carbon dioxide and water combine to fom the products oxygen and sugar. The reaction takes place in the leaves of green plants where chlorophyll is present and is driven by light energy from the Sun. 12. In very cold climates, the amount of snow on the ground may decrease even if the temperature stays below 0 degrees Celsius. What process is most likely responsible for this event? Sublimation 13. Compounds have properties different from their individual elements. The minimum number of elements needed to combine to form a compound is TWO. All compounds have chemical names and many have common names as well. The chemical name indicates which elements make up the compound. Sometimes prefixes are used to indicate the number of atoms in a compound. Mon – means one; di or bi means two; tri means three. Carbon dioxide has one carbon and two oxygen atoms. Scientists describe compounds by combining letters and numbers into a chemical formula. Chemical formulas indicate with abbreviations the elements that are in the compound, and in what ratio. A chemical equation keeps track of which substances are used, and in what ratio. The number in front of the chemical formula tells how many of these molecules are used. The chemicals on the left side of a chemical reaction are called reactants. The chemicals on the right side of the equation are the products. 14. Chemical reactions occur when atoms link together in new ways to create substances different from the original substances. This is also known as a chemical change. 15. Examples of chemical reactions: mixing baking soda and peroxide; burning paper; food cooking; baking bread; rust; tarnish; burning candle 16. If 32 atoms of hydrogen react completely with 16 atoms of oxygen, how many molecules of water will be made? Why? 16, because each water molecule has one oxygen atom and two hydrogen atoms 17. Signs of a chemical change: change in color, release of gas, the appearance of bubbles, formation of a precipitate (a solid formed from the chemical reaction of some solutions), a release of energy like light or heat 18. When corrosion of a metal causes a color change, it is called tarnish. 19. When you eat and breathe, you reverse photosynthesis. The reverse reaction is called respiration. Your body cells force glucose and oxygen to rearrange atoms back into carbon dioxide and water. This releases the stored energy for you to use to live. The reactants of photosynthesis are carbon dioxide and water, and the products are glucose and oxygen. The reactants of respiration are glucose and oxygen, and the products are carbon dioxide and water. 20. What are some chemical reactions that affect your life? Using oil or gas to produce heat or mechanical energy; cooking; almost everything you buy or use is the result of a chemical reaction **There will be a Bonus on balancing equations to make sure the law of conservation holds true.