ELEMENTS and COMPOUNDS SUMMARY ANSWERS
advertisement

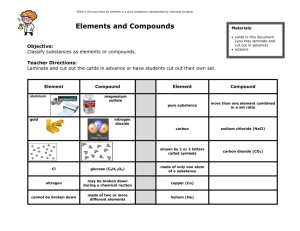
ELEMENTS and COMPOUNDS SUMMARY ANSWERS Answer the following in your jotter. Elements 1. Everything is made from atoms or molecules. 2. a) An element is made up of only one kind of atom. b) There are about 100 elements. c) The elements are listed in the Periodic Table. d) Metals are found on the left of this table. Non metals are found on the left/right side of this table. e) H – Hydrogen C – Carbon Na – Sodium Cu – Copper Al – Aluminium Classifying Elements 3. Alkali metals, very reactive 4. Alkaline earth metals, reactive 5. Halogens, reactive 6. Any transition metals Mixtures 7. A mixture can be separated easily. Compounds 8. A compound is formed when two or more elements chemically join. 9 . The difference is mixtures can be separated easily whereas compounds can not, since in a compound the elements are joined, but in a mixture they are not. 10. Copy and complete the following table. Compound Elements present Magnesium oxide Magnesium, oxygen Sodium chloride Sodium, chlorine Potassium fluoride Potassium, fluorine Aluminium sulphide Aluminium, sulphur Gases 11a) Nitrogen – about 80% b) Oxygen – about 20% c) Argon – about 1% d) Carbon dioxide – about 0.03% 12. Write down the tests for the following gases; a) Hydrogen – burns with a pop b) Oxygen – relights a glowing splint c) Carbon dioxide – turns lime water milky/cloudy Splitting up compounds 13. Electrolysis – splits the compound using electricity. 3. Gases 1. a) Nitrogen – about 80% b) Oxygen – about 20% c) Argon – about 1% d) Carbon dioxide – about 0.03% 2. a) Nitrogen – making fertilisers, liquid nitrogen is used to freeze food. b) Oxygen – Breathing, producing hot flames for welding. c) argon – filling light bulbs d) Carbon dioxide – fire extinguishers, the fizz in fizzy drinks. Burning 1) Oxygen is used up when something burns. 2) The following are flammable materials Petrol, paper, natural gas etc