CP Chem
advertisement

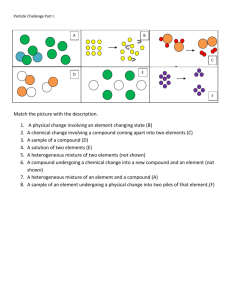
Worksheet #1 – Classification of Matter I Choose words from the list to fill in the blanks in the paragraphs. chemical property mixture extensive property substance intensive property element property homogenous matter compound physical property heterogeneous matter Matter has uniform characteristics throughout is called (1) ____. Matter that has parts with different characteristics is called (2). A characteristic by which a variety of matter is recognized is called a(n) _______(3)__________. A characteristic that depends upon the amount of matter in the sample is called a(n) ____(4)________. A characteristic that does not depend upon the amount of matter is called a(n) _________(5)________. A characteristic that can be observed without producing new kinds of matter is called a(n) ________(6)______. A characteristic that depends on how a kind of matter changes during interactions with other kinds of matter is called ________(7)________. Matter can also be classified according to the basic types of matter it contains. A simple substance that cannot be broken down into other substances by chemical means is called _______(8)_______. A chemical combination of simple substances is called ________(9)______. A physical combination of different substances that retain their individual properties is called a(n) ________(10)_______. Either an element or a compound may be referred to as a(n) _______(11)______. 1. ________________ 2. ________________ 3. ________________ 4. ________________ 5. ________________ 6. ________________ 7. ________________ 8. ________________ 9. ________________ 10. ________________ 11. ________________ MIXTURES Classify each of the following as an element, compound, heterogeneous mixture, or homogenous mixture, solution, alloy. 12. 14. 16. 18. 20. 22. 24. 26. Water _______________ Air _______________ Sugar dissolved in water _______________ Homogenized milk _______________ Sand in water _______________ Selenium ________________ baking powder ___________ Apple___________ 13. 15. 17. 19. 20. 23. 25. 27. Carbon _______________ Table salt, NaCl _______________ Granite _______________ Oxygen _______________ concrete ____________ brass ______________________ hydrogen sulfide _____________ bronze ___________________ Classify each of the following substances as: an element, a compound, a solution (which is a homogenous mixture), or a heterogeneous mixture. 1) Sand 2) Water 3) Pure Water 4) Soil 5) Soda 6) Pure Air 7) Carbon Dioxide 8) Gold 9) Bronze 10) Oxygen 11) Salad Dressing 12) Salt Water 13) KoolAid 14) Chocolate Milk 15) Coffee with Milk 16) Salt 17) Steel 18) Calcium 19) Raisin Bran 20) Gasoline In the spaces provided, describe the distinguishing characteristics of the major categories of matter. 21. Element - 22. Compound - 23. Solution (homogenous) - 24. Mixture (heterogeneous)-