Determination of the hydration enthalpy of an electrolyte with Cobra 4
TEC
Related topics
Integral enthalpy of solution, Hess’s law, lattice energy, ion solvation, calorimetry.
Principle
When a solid electrolyte dissolves in water, a positive or negative heat effect occurs as a result of the
destruction of the crystal lattice and the formation of hydrated ions. The enthalpy of hydration of copper
sulphate can be calculated from the different heats of reaction measured when anhydrous and hydrated
copper sulphate are separately dissolved in water.
Equipment
2
1
1
1
1
1
1
4
1
1
1
1
1
1
Cobra4 USB-Link
Cobra4 Sensor-Unit Energy
Cobra4 Sensor-Unit Temperature
Software measure Cobra4
Calorimeter, transparent
Heating coil with sockets
Universal power supply
Connection cable, l =500 mm, black
Magnetic heating stirrer
Magnetic stirrer bar, l = 30 mm, oval
Separator for magnetic bars
Support rod, l = 500 mm, M10 thread
Right angle clamp
Universal clamp
1 Set of Precision Balance Sartorius CPA
6202S and measure software, 230 V
1 Mortar with pestle, 190 ml
1 Spoon, special steel
12610-00
12656-00
12640-00
14550-61
04402-00
04450-00
13500-93
07361-05
35751-93
35680-04
35680-03
02022-20
37697-00
37715-00
49226-88
32604-00
33398-00
Porcelain dish, 115 ml, d = 100 mm
Crucible tongs, 200 mm
Tripod, d = 140 mm, h = 240 mm
Wire gauze, 160 x 160 mm
Butane burner
Butane cartridge
Powder funnel, d = 100 mm
Glass beaker, 50 ml, tall
Wash bottle, 500 ml
Desiccator
Porcelain plate for desiccators
Silicone grease, 50 g
Silica gel, orange, granulated, 500 g
Copper(II) sulphate, anhydride, 250 g
Copper(II) sulphate, 250 g
32518-00
33600-00
33302-00
33287-01
32178-00
47535-00
34472-00
36001-00
33931-00
34126-00
32474-00
31863-05
30224-50
31495-25
30126-25
1 Water, distilled, 5 l
Additional equipment
PC, with USB interface, Windows®XP
or higher
31246-81
1
1
1
1
1
1
1
2
1
1
1
1
1
1
1
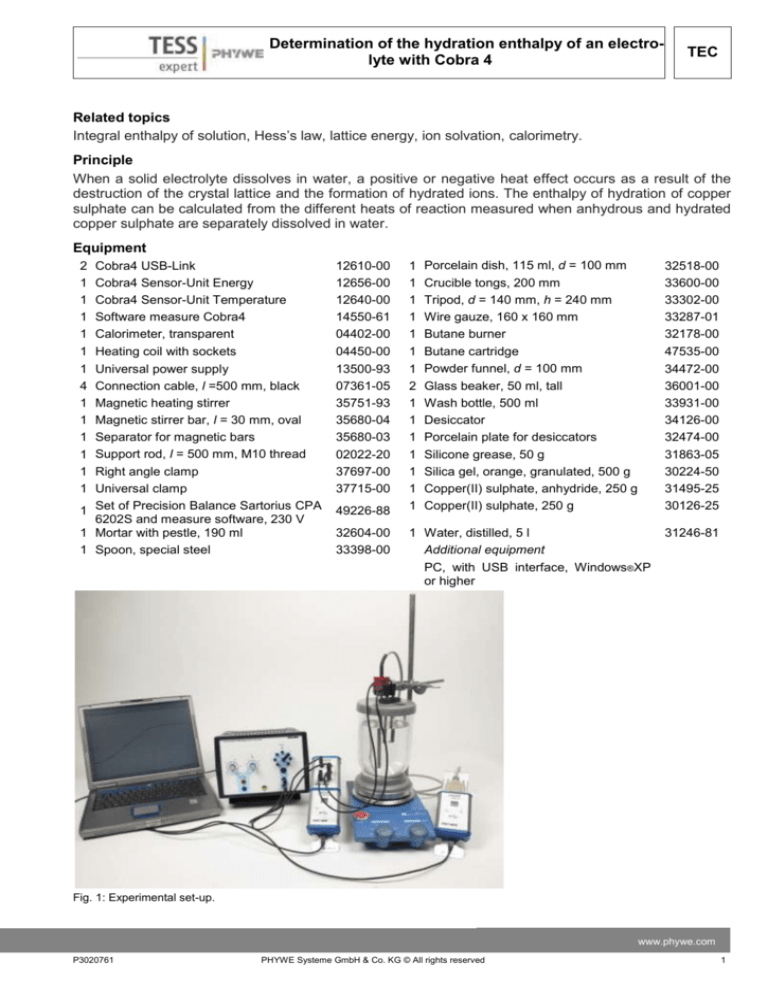
Fig. 1: Experimental set-up.
www.phywe.com
P3020761
PHYWE Systeme GmbH & Co. KG © All rights reserved
1
TEC
Determination of the hydration enthalpy
of an electrolyte with Cobra 4
Safety instructions
When handling chemicals, you should wear suitable protective gloves, safety goggles, and suitable
clothing. Please refer to the appendix for detailed safety instructions.
Tasks
1. Record temperature-time curves for the dissolution of anhydrous copper sulphate and hydrated copper sulphate in water.
2. Calculate the hydration enthalpy of anhydrous copper(II)sulphate.
Set-up and procedure
Set up the experiment as shown in Fig.1.
Combine the Cobra4 Sensor-Unit Energy and the Cobra4 Sensor-Unit Temperature with the Cobra4
USB-Links.
Connect the power supply with the Cobra4 Sensor-Unit Energy but leave the heating coil unconnected to
Cobra4 Sensor-Unit Energy.
Start the PC and connect each Cobra4 USB-Link with a USB socket of the computer.
Call up the ‘measure’ programme in Windows.
Some ID numbers (01 and 02) are allocated to the sensors, which are indicated in the displays of the
Cobra4 USB-Links.
Prepare the two copper salts by grinding each of them separately to a fine powder in a mortar.
Make sure that the anhydrous copper sulphate really is anhydrous by heating it in a porcelain dish over a
butane burner until it is completely white and allowing it to cool in a desiccator. Weigh 24.97 g (0.1 mol)
of copper(II) sulphate and 15.96 g (0.1 mol) of anhydrous copper(II) sulphate in two separate beakers
(weighing accuracy 0.01 g). Fill the calorimeter with 900 g of distilled water (weighing accuracy 0.1 g).
Put the oval magnetic stirrer bar into the calorimeter and switch on the magnetic stirrer (Caution: Do not
mistakenly switch on the heating unit!).
Wait until temperature equilibrium has been reached (approximately 10 min). Start the measurement
with a click on
in the icon strip. Wait 3 to 4 minutes, then add the first copper salt to the water by pouring it through the powder funnel which has been inserted in the opening of the lid. Make sure that the entire quantity of salt is added to the water without any loss.
Continue to measure until a new equilibrium has been reached. Now, perform electrical calibration to determine the total heat capacity of the calorimeter. To do this, supply 10 V AC to the Cobra4 Sensor-Unit
Energy for the electric heating and then put the free ends of the heating coil connection cables into the
output jacks. The system is now continuously heated and the supplied quantity of energy is measured.
When approximately 4000 Ws are transferred, switch off the heating by pulling the connection cables out
of the heating coil. Continue to measure for another three minutes, then stop temperature recording with
a click on in the icon strip.
2
PHYWE Systeme GmbH & Co. KG © All rights reserved
P3020761
Determination of the hydration enthalpy of an electrolyte with Cobra 4
TEC
Repeat the experiment to determine the enthalpy of solution of the second copper salt. At least two
measurements for each salt should be performed to avoid errors and to calculate a mean value.
Theory and evaluation
The dissolving of a solid electrolyte in water is primarily determined by two simultaneously occurring processes: the destruction of the crystal lattice and the hydration of the ions. The destruction of the crystal
lattice is an endothermic process because energy is required to break down the chemical bonds, whereas the hydration of the ions is exothermic. Depending on the type of lattice, and on both the radius and
the charge of the ions (charge density), the resulting enthalpy of solution can be either endothermic or
exothermic.
When a salt exists in both hydrated and dehydrated forms, and on assuming that when the hydrated salt
dissolves only the degradation of the crystal lattice occurs, the enthalpy of hydration can be calculated
using Hess’s theorem.
Fig. 2: Temperature-time curve of solution of copper (II) sulphate and determining the heat capacity of the system.
www.phywe.com
P3020761
PHYWE Systeme GmbH & Co. KG © All rights reserved
3
Determination of the hydration enthalpy
of an electrolyte with Cobra 4
TEC
Fig. 3: Temperature-time curve of solution of anhydrous copper (II) sulphate and determining the heat capacity of the system.
𝚫𝐋 𝑯 =
𝚫𝐋 𝒉
𝒏
𝚫𝐇 𝑯𝐂𝐮𝐒𝐎𝟒 = 𝚫𝐋 𝑯𝑪𝒖𝑺𝑶𝟒 − 𝚫𝐋 𝑯𝐂𝐮𝐒𝐎𝟒∙𝟓 𝐇𝟐𝐎
𝚫𝐇 𝑯
𝚫𝐋 𝑯
𝚫𝐋 𝒉
Enthalpy of hydration
Molar enthalpy of solution
Integral enthalpy of solution
The integral enthalpy of solution can be calculated according to equation (3)
𝚫𝐋 𝑯 =
𝐐𝐞𝐱𝐩
𝒏
𝑸𝐞𝐱𝐩 = 𝑸𝐜𝐚𝐥 ∙
4
(2)
𝚫𝑻𝐞𝐱𝐩
𝚫𝑻𝐜𝐚𝐥
(3)
PHYWE Systeme GmbH & Co. KG © All rights reserved
P3020761
Determination of the hydration enthalpy of an electrolyte with Cobra 4
𝑸𝐞𝐱𝐩
𝑸𝐜𝐚𝐥
𝚫𝑻𝐞𝐱𝐩
𝚫𝑻𝐜𝐚𝐥
𝒏
TEC
Heat of solution of a salt
Electrical work for calibration
Temperature difference during the dissolution of the salt
Temperature difference during the calibration
Quantity of salt
Data and results
𝑴𝐂𝐮𝐒𝐎𝟒
= 159.6 g/mol
𝑴𝐂𝐮𝐒𝐎𝟒∙𝟓 𝐇𝟐𝐎
= 249.68 g/mol
𝚫𝐋 𝑯𝐂𝐮𝐒𝐎𝟒
= -66.2 kJ/mol
𝚫𝐋 𝑯𝐂𝐮𝐒𝐎𝟒∙𝟓 𝐇 𝐎
= +11.5 kJ/mol
𝟐
𝚫𝐇 𝑯𝐂𝐮𝐒𝐎𝟒
= -77.7 kJ/mol
Appendix
Hazard symbol, signal word
Hazard statements
Precautionary statements
Copper(II) sulphate
P273: Avoid release to the environment
H302: Harmful if swallowed
P305+351+338: IF IN EYES:
H315: Causes skin irritation
Rinse cautiously with water for
H319: Causes serious eye irrita- several minutes. Remove contact
tion
lenses if present and easy to do.
H410: Very toxic to aquatic life Continue rinsing
with long lasting effects
P302+252: IF ON SKIN: Wash with
soap and water.
Attention
Copper(II) sulphate anhydrite
Attention
P273: Avoid release to the environment
H302: Harmful if swallowed
P305+351+338: IF IN EYES:
H315: Causes skin irritation
Rinse cautiously with water for
H319: Causes serious eye irrita- several minutes. Remove contact
tion
lenses if present and easy to do.
H410: Very toxic to aquatic life Continue rinsing
with long lasting effects
P302+252: IF ON SKIN: Wash with
soap and water.
www.phywe.com
P3020761
PHYWE Systeme GmbH & Co. KG © All rights reserved
5
TEC
Determination of the hydration enthalpy
of an electrolyte with Cobra 4
Room for notes:
6
PHYWE Systeme GmbH & Co. KG © All rights reserved
P3020761









