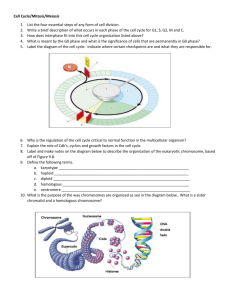
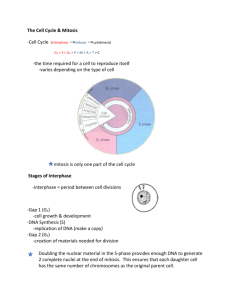
exercise questions
advertisement

Laboratory Resource Guide to accompany Essentials of Biology Laboratory Manual Fourth Edition Sylvia S. Mader 1 Scientific Method Unit I The Cell 2 Measuring with Metric 3 Microscopy 4 Cell Structure and Function 5 Enzymes 6 Photosynthesis Unit II Genetics 7 Cellular Reproduction 8 Sexual Reproduction 9 Patterns of Inheritance 10 DNA Biology and Technology 11 Genetic Counseling Unit III Evolution and Diversity of Life 12 Evidences of Evolution 13 Microbiology 14 Plant Evolution 15 Plant Anatomy and Growth 16 Animal Evolution Unit IV Animal Structure and Function 17 Basic Mammalian Anatomy I 18 Chemical Aspects of Digestion 19 Energy Requirements and Ideal Weight 20 Basic Mammalian Anatomy II 21 Nervous System and Senses Unit V Ecology 22 Effects of Pollution on Ecosystems 1 Laboratory 1 Scientific Method (LM pages 1–8) Fourth Edition This lab has been much improved in a number of ways. In Section 1.1, examples are provided for each step of the scientific method and in Section 1.3 and 1.4, students use plus and minus signs to hypothesize and then record a pillbug's reaction to test substances. Table 1.4 has an improved design for collecting class data. New/Revised Figure. 1.1 Pillbugs on leaf New/Revised Tables. 1.1 Pillbug Speed; 1.2 Hypotheses About Pillbug's Response to Potential Foods; 1.3 Pillbug's Response to Potential Foods; 1.4 Pillbug's Response to Potential Foods: Class Results MATERIALS AND PREPARATIONS Instructions are grouped by procedure. Some materials may be used in more than one procedure. Special Requirements Living material. Live pillbugs, Armadillidium vulgare, for all sections of lab Earthworm alternative. Refer to the section titled “Earthworm Alternative” at the end of this laboratory if you wish to use earthworms instead of pillbugs. Fresh material. Substances for instructor to feed pillbugs and substances for students to test pillbug behavior are listed in Section 1.4. 1.2 Observing a Pillbug (LM pages 4–5) _____ pillbugs, Armadillidium vulgare, live (Carolina 14-3082) _____ pen, white (or correction fluid, white) or taped tags _____ magnifying lenses or stereomicroscopes _____ small glass or plastic dishes, such as disposable Petri dishes _____ graduated cylinders or small beakers for observing pillbug movement _____ rulers, metric, 30 cm plastic _____ stopwatch Pillbugs. If ordering, ask for 50 pillbugs for a class of 20 to 35 or more students. Order pillbugs so that they arrive as close as possible to the date of use. Follow care and feeding instructions provided with the pillbug order and/or see the following. 2 Collecting pillbugs (LM pages 1, 4–7). Pillbugs like moisture and avoid sunlight. They can be found next to brick buildings along the grass line or next to sidewalks, or under logs and planks of wood. They are attracted to wet grass covered with a cardboard box or plastic tarp. Encourage students to collect their own pillbugs and give them lab participation points. Collect pillbugs in the spring, summer, and fall as they are hard to find in the winter. After collecting, pillbugs can be easily maintained in a terrarium to keep a fresh supply all year long. They feed primarily on decaying organic matter; they like moisture and avoid sunlight. They like carrots and cucumbers. Change the food daily to prevent mold growth. Preparation for lab. Withdraw food 1–2 days prior to the experiment. Use white correction fluid or tape tabs to number the pillbugs for identification. 1.4 Performing the Experiment and Coming to a Conclusion (LM pages 6–7) _____ pillbugs, Armadillidium vulgare, live (Carolina 14-3082) _____ small beakers, 35-mm film cans, watch glasses, or small Petri dishes for distributing test substances _____ Petri dishes, preferably 150 mm (or else 100 mm) for testing the pillbugs _____ small plastic bottle for spritzing _____ distilled water _____ cotton balls Suggested test substances: _____ flour _____ cornstarch or brand flakes _____ coffee creamer _____ baking soda _____ fine sand (control) _____ milk _____ orange juice or apple juice _____ ketchup _____ applesauce _____ carbonated beverage _____ water (control) Do not use salt, vinegar, or honey, as these substances are harmful to pillbugs. Plain water is used as a control for liquids. Fine sand is used as a control for powders. Experimental design (LM pages 6–7). These methods are recommended: For a dry substance, make a circle of the test substance in a Petri dish and put the pillbug in the center of the circle. For a liquid, put a cotton ball soaked with the test substance in the pillbug's path. Rinse pillbugs between testing procedures by spritzing with distilled water and then placing them on a paper towel to dry. Suggestions. The experiment goes well and clean up is easier if there is a limited number of test substances and each student chooses only two dry and two liquid test substances. 3 Substances can be distributed to several stations in small beakers, 35-mm film cans, watch glasses, or small Petri dishes. Testing pillbugs in 150 mm Petri dishes works well. EXERCISE QUESTIONS 1.1 Using the Scientific Method (LM pages 2–3) Why does the scientific method begin with observations? To study the natural world, scientists have to observe natural phenomena. What is the benefit of formulating a hypothesis? The hypothesis tells what is to be tested by experiment or further observations. Why must a scientist keep a complete record of an experiment? So others can repeat the experiment and can check that the data are valid. What is the purpose of the conclusion? The conclusion tells whether the hypothesis was supported or not. How is a scientific theory different from a conclusion? Each experiment has a conclusion. A scientific theory is based on many conclusions from various experiments in related fields. 1.2 Observing a Pillbug (LM pages 4–5) Observation: Pillbug’s External Anatomy (LM page 4) 1. How can you recognize the head end of a pillbug? The head bears antennae and eyes. How many segments and pair of walking legs are in the thorax? There are 7 segments and 7 pairs of legs. Observation: Pillbug’s Motion (LM page 5) 1. a. Describe the action of the feet and any other motion you see. The seven pairs of legs move with the front pair leading, and each pair moves in succession thereafter. b. Allow a pillbug to crawl on your hand. Describe how it feels. It tickles the skin as it moves. c. Does a pillbug have the ability to move directly forward? yes d. Do you see evidence of mouthparts on the underside of the pillbug? A pillbug has four pairs of mouthparts. 2. As you watch the pillbug, identify a. the anatomical parts that allow a pillbug to identify and take in food. antennae, eyes, and mouthparts b. behaviors that will allow a pillbug to acquire food. For example, is the ability of a pillbug to move directly forward a help in acquiring food. Explain. Yes, because it is the most efficient way to reach food. What other behaviors allow a pillbug to acquire food? A pillbug has appropriate mouth parts for taking in and eating food. c. a behavior that helps a pillbug avoid dangerous situations. The pillbug rolls into a ball when it is threatened. 4 Table 1.1 Pillbug 1 2 3 Pillbug Speed* Millimeters (mm) Traveled Time (sec) Speed (mm/sec) 71 30 2.36 122 60 2.20 64 30 2.12 Average speed: 2.23 mm/sec *Answers will vary. The answers provided here are examples. 1.3 Formulating Hypotheses (LM page 6) 1-3. See Table 1.2 showing three possible student hypotheses regarding flour. Students uses "0" for no response, "—" for moves away from the substance, and "+" for moving toward the substance and eating it. Table 1.2 Hypotheses about Pillbug’s Response to Potential Foods Substance Hypothesis About Reason for Hypothesis Pillbug’s Response to Potential Foods Flour 0 Flour is a bland substance. Flour — Flour is a dry substance. Flour + Flour is a food substance. 1.4 Performing the Experiment and Coming to a Conclusion (LM pages 6–7) Experimental Procedure: Pillbug’s Response to Potential Foods (LM pages 6–7) Table 1.3 Pillbug’s Response to Potential Foods Substance Pillbug’s Response Hypothesis supported? Flour + Depends on hypotheses Cornstarch + Coffee creamer + Baking soda — Fine sand 0* Milk + Orange juice — Ketchup — Applesauce + Carbonated beverage + Water 0* *Pillbugs may move toward these substances but do not eat them. 5. Do your results support your hypotheses? depends on results 6. Are there any hypotheses that were not supported by the experimental results (data)? How do such data give you more insight into pillbug behavior? Explain. Answer depends on student hypotheses. 5 Table 1.4 Pillbug's Response to Potential Foods: Class Results Answers will vary depending on class data. 8. On the basis of the class data do you need to revise your conclusion for any particular pillbug response? depends on class data Why is this the best methodology? The more trials, the more likely the results are valid. 9. Did the billbugs respond as expected to the controls, i.e., did not eat them. depends on student results If they did not respond as expected, what can you conclude about your experimental results? The results may be invalid. LABORATORY REVIEW 1 (LM page 8) 1. What are the essential steps of the scientific method? making observations, formulating a hypothesis, testing the hypothesis, coming to a conclusion 2. What is a hypothesis? tentative explanation of observed phenomena 3. Is it sufficient to do a single experiment to test a hypothesis? No, because reliability increases the number of times the experiment is repeated and the results remain the same. 4. What do you call a sample that goes through all the steps of an experiment and does not contain the factor being tested? control 5. What part of a pillbug is for protection and what does a pillbug do to protect itself? exoskeleton. A pillbug rolls into a ball to protect itself. 6. State the type of data you used to formulate your hypotheses regarding pillbug reactions toward various substances. Observational data. 7. Why is it important to use one substance at a time when testing a pillbug’s reaction? only then can you be certain of the pillbug’s reaction to that particular substance Indicate whether statements 8 -10 are hypotheses, conclusions, or scientific theories: 8. The data show that vaccines protect people from disease. conclusion 9. All living things are made of cells. theory 10. The breastbone of a chicken is proportionately larger than that of any other bird. hypothesis Earthworm Alternative Earthworms can be used instead of pillbugs for all of the exercises in this laboratory. Place earthworms in large rectangular plastic storage containers and let them roam around for approximately 15 min. These containers can also be used to keep earthworms between 6 experiments. Plexiglass is also needed to place test substances on while holding earthworms above to see behavior towards substances. Earthworms want to move rapidly to escape. They are inclined to move away from light, move under things, and seem to want to move downward. They are expected to move away from a heat source. They also move toward each other and pile up on each other. They can move up and down on glass at a 45 degree angle. With regard to what students already know about earthworm activity, they might predict certain behaviors. Earthworms live (or hide) in the soil, so they would move down and through soil. Soil prevents desiccation and keeps them cool and moist. By moving under things, they could stay cooler, stay moist, and stay hidden in the dark. Perhaps light bothers them also. Earthworms can move backward and forward from both ends. When they are investigating a substance, they make a long, skinny point out of the end they are investigating with, and if they are repelled by a substance, they pull back and the end becomes thick and round. When testing with liquids, if an earthworm gets even close to the substance, the substance will be pulled along the earthworm’s body without the earthworm doing anything. Capillary action or cohesion tension? To prevent this, hold the earthworm above the substance, in case the substance (especially lemon juice) might harm the earthworm. Just let the worm move its pointed end into or near the substance. You can tell when it is repelled as it will pull away. Rinse the earthworm right away if it touches a substance (especially lemon juice). WHEN FINISHED WITH EARTHWORMS, mix damp potting soil with some oatmeal, potato peels, lettuce, or other organic matter from the test—not too much, just enough to give the earthworms something to eat. Add earthworms. Cover container with newspaper. Keep soil damp. When completely finished, release earthworms into garden or greenhouse soil. 7 Laboratory 2 Measuring With Metric (LM pages 9-18) Fourth Edition This is a new lab that teaches the metric system. MATERIALS AND PREPARATIONS Instructions are grouped by exercise. Some materials may be used in more than one exercise. 2.1 Length (LM pages 10–12) _____ meter stick, metric and English _____ long bones from disarticulated human skeleton _____ cardboard (10 cm 30 cm), two pieces _____ rulers, plastic millimeter 2.2 Weight (LM pages 13–14) _____ sturdy balance scale _____ wooden block, small enough to hold in hand _____ object, such as a piece of granite, or a trilobite fossil, small enough to fit through the opening of a small graduated cylinder _____ triple beam balance _____ objects to weigh: penny, paper clip, quarter 2.3 Volume (LM pages 14–15) _____ wooden block and object from above _____ graduated cylinders, 50 ml or 100 ml _____ test tubes (large enough to hold 20 ml of water) _____ dropper bottles containing water _____ index card, blank white (20 cm × 30 cm) _____ beaker, 50 ml _____ graduated pipette (for demonstration) 2.4 Temperature (LM page 16) _____ thermometer, Celsius _____ cold water, hot water, ice water 8 EXERCISE QUESTIONS 2.1 Length (LM pages 10–12) You will want to know what these abbreviations stand for, so write them out here: m = meter µm = micrometer cm = centimeter nm = nanometer mm = millimeter How many cm are in a meter? 100 How many mm are in a centimeter? 10 How many µm are in a millimeter? 1,000 How many nm are in a micrometer? 1,000 Meter, Centimeter, and Millimeter (LM pages 10–11) Observation: A Meterstick (LM pages 10–11) 2. How many centimeters are in a meter? 100 For example, The prefix centi- means 100. For example, how many cents are in a dollar? 100 3. How many millimeters are in a centimeter? 10 But the prefix milli- means a thousand. How many millimeters are in a meter? 1,000 Obtain a penny and measure its width in terms of mm. 18 mm Why does it seem preferable to measure a penny in terms of millimeters? to use whole numbers 4. For example, if the bone measures from the 22 cm mark to the 50 cm mark, the length of the bone is 28 cm. If the bone measures from the 22 cm marks to midway between the 50 cm and 51 cm marks, its length is 27.5 cm = 285 mm. 5. Record the length of two bones. Recorded lengths will vary. Millimeter, Micrometers, and Nanometer (LM pages 11–12) Observation: Small Metric Ruler (LM page 12) 1. Use the ruler to measure the diameter of the circle shown to the nearest mm. Diameter of circle in mm: 38 mm 2. Do you expect the answer to be a smaller number or larger number? larger Size of the circle in µm: 38,000 µm 3. Diameter of circle in nm: 38,000,000 nm 4. You have shown that the diameter of the circle is 38 mm = 38,000 µm = 38,000,000 nm. 2.2 Weight (LM pages 13–14) Using a revision of the formula on page 12 if necessary, do these conversions: 2g = 2,000 mg and 0.2 g = 200 mg. Experimental Procedure: Weight (LM pages 13–14) 1. The weight of the wooden block is (Answers will vary.). 2. Measure the weight of an item small enough to fit inside the opening of a 50 ml graduated cylinder. Answers will vary. 3. If so directed by your instructor, use a triple beam balance to take the weight of one or more of these objects to a tenth of a gram: Penny 2.5 g Paper clip 1.4 g Quarter 4.7 g 9 2.3 Volume (LM pages 14–15) Experimental Procedure: Volume (LM pages 14–15) 1. For example, use a millimeter ruler to measure the wooden block used in the previous Experimental Procedure to get its length, width, and depth. Answers will vary according to the size of the block used. Computations of volume will also vary. 3. Hypothesize how you could find the total volume of the test tube. Fill the test tube with water, and pour the water into the graduated cylinder. Read the volume in milliliters. What is the test tube’s total volume? Answers will vary. 4. Hypothesize how you could use this setup to calculate the volume of the small object you weighed previously (see step 2, p. 13). Fill the cylinder with water to the 20 ml mark. Drop the object into the cylinder, and read the new elevated volume. The difference between the two readings is the volume of the object alone. Now perform the operation you suggested. Answers will vary. 5. Hypothesize how you could determine how many drops from the pipette of the dropper bottle equal 1 ml. Using a 10 ml graduated cylinder, count the number of drops it takes to get to 1 ml. How many drops from the pipette of the dropper bottle equal 1 ml? approximately 10 (Answers will vary with student’s technique and with the type of pipette.) 6. Are pipettes customarily used to measure large or small volumes? small 2.4 Temperature (LM page 16) Experimental Procedure: Temperature (LM page 16) 1. a. Water freezes at 32°F = 0°C. b. Water boils at 212°F =100°C. 2. Human body temperature of 98°F is what temperature on the Celsius scale? 37°C 3. Record any two of the following temperatures in your lab environment. Answers will vary. 2.5 Summary (LM page 17) In Table 2.2. except for centimeter, the units are all 10X larger than the next unit. So, when you convert a gram to milligram, you multiple the gram by 1000. To determine what portion (milligrams to gram) you divide by 1000. LABORATORY REVIEW 2 (LM pages 18) 1. What type of measurement is signified by kg? weight ml? volume cm? length 2. 3. 4. 5. degrees? temperature μm? length What type of measurement would utilize a meterstick? length a graduated cylinder? volume a balance scale? weight If a triple beam balance shows a weight of 100 g plus 10 g plus 1 g, what is the weight of the object? 111 g An object is added to a graduated cylinder that holds 250 ml, and the water rises to 300 ml. What is the volume of the object in ml? 50 ml in cm3 ? 50 cm3 Name two units of measurement you expect to use in the next laboratory, 10 which concerns microscopy? micrometer (μm) and nanometer (nm). 6. How many micrometers are in a millimeter? 1,000 Convert 1.1 mm to μm. 1,100 μm 7. How many milliliters are in a liter? 1,000 Convert 500 ml to liters. 0.5 0 l 8. How many milligrams are in a gram? Convert 5 g to mg. 1,000 5,000 mg 9. Convert 1.5 cm to μm. Show your work. 1.5 cm = 15 mm= 15,000 μm 10. A student looking for a shortcut drops an object in a graduated cylinder that contained water to find its weight. What’s wrong? and not weight. To measure weight use a scale. This will measure volume 11 Laboratory 3 Microscopy (LM pages 19-30) Fourth Edition This laboratory now contains only microscopic study as the metric system is now laboratory 2. MATERIALS AND PREPARATIONS Instructions are grouped by exercise. Some materials may be used in more than one exercise. Special Requirements Living material. Euglena. Order two weeks before laboratory. Notes Microscope supplies. Set aside an area in the lab for storage of clean microscope slides, coverslips, and lens paper. Post a notice in this area, outlining the established procedures for handling dirty slides. Possible procedures include: 1. Wash, rinse, and dry all slides, and return them to their boxes or place them in the drying rack. 2. To wash, place dirty slides in the detergent solution provided; discard plastic coverslips. Glass coverslips should be placed in detergent solution in a beaker. 3. Some laboratories prefer that the laboratory assistant wash all slides in an ultrasonic cleaner, rinse the slides in distilled water, and allow the slides to drain dry. 3.2 Stereomicroscope (Binocular Dissecting Microscope) (LM pages 22–23) _____ microscope, stereomicroscope with illuminator _____ lens paper _____ an assortment of objects for viewing (e.g., coins, plastomount) 3.3 Use of the Compound Light Microscope (LM pages 24–27) _____ microscopes, compound light _____ lens paper _____ slide, prepared: letter e or newspaper, scissors, slides, and coverslips _____ rulers, clear plastic millimeter _____ slide, prepared: colored threads or to prepare your own, you will need slides and coverslips, three or four colors of sewing thread (or hairs), scissors, and a dropping bottle of water 3.4 Microscopic Observations (LM pages 27–29) _____ microscope slides (glass or plastic) _____ covers slips 12 _____ _____ _____ _____ _____ _____ _____ _____ _____ _____ _____ lens paper microscopes, compound light toothpicks, prepackaged flat biohazard waste container for toothpicks ethyl alcohol (ethanol), 70%; or alcohol swabs (if toothpicks are not prepackaged) Euglena onion optional prepared slide: human stratified squamous epithelium, cheek methylene blue solution, or iodine-potassium-iodide (IKI) solution (premade)) dropping bottles, or bottles with droppers Protoslo® or methyl cellulose solution Methylene blue solution (LM page 28). Make up a 1.5% stock solution, using 1.5 g methylene blue stain in 100 ml of 8.5% ethyl alcohol (ethanol, Carolina 86-1281). Dilute one part stock solution with nine parts water for laboratory use, or use iodine (IKI) solution. Methylene blue staining solution can also be purchased premade. Iodine (IKI) solution (LM page 28). Iodine-potassium-iodide (IKI) solution can be purchased premade, or the ingredients can be purchased separately as potassium iodide (KI) and iodine (I). These dry ingredients have a long shelf life and can be mixed as needed, according to the following recipe: To make a liter of stock solution, add 20 g of potassium iodide (KI) to 1 liter of distilled water, and stir to dissolve. Then add 4 g of iodine crystals, and stir on a stir plate; dissolution will take a few hours or more. Keep the stock reagent in dark, stoppered bottles. For student use, place in dropping bottles. Label as “iodine (IKI) solution.” Iodine solution stored in clear bottles loses potency over time. If the solution lightens significantly, replace it. Small dropper bottles can be stored for about a month, and they are used in other exercises. A screw-capped, brown bottle of stock iodine can be stored for about six months. Dispose of it if the solution turns light in color. Human epithelium cheek slide (LM page 28). To eliminate the possibility of contact with pathogens, this exercise can be done as a demonstration using a flexscope or videoscope for students to view from their seats. Otherwise, because of the hazards connected with human tissue samples and body fluids, you should take special precautions if students are preparing their own epithelium slides. Use a biohazardous waste container for toothpick disposal, and wash slides and coverslips in a 10% bleach solution. Microscopes should also be wiped with a disinfecting solution. Dropping bottles. Various styles of dropping bottles are available—for example, dropper vials, glass screw-cap (Carolina 71-6438, -6434) with attached droppers; Barnes dropping bottles (Carolina 71-6525); and plastic dropping bottles (Carolina 71-6550). See also Carolina’s Laboratory Equipment and Supplies section. 13 Protoslo® (or methyl cellulose solution) (LM page 29). You can also use glycerol (Carolina 86-5530) and water as a substitute for Protoslo®. Note: Thickened Protoslo® can be reconstituted with distilled water. EXERCISE QUESTIONS 3.1 Light Microscopes Versus Electron Microscopes (LM pages 20-21) Answer These Questions (LM page 21) Which two types of microscopes view the surface of an object? stereomicroscope and scanning electron microscope Which two types of microscopes view objects that have been sliced and treated to improve contrast? compound light microscope and transmission electron microscope Of the microscopes just mentioned, which one resolves the greater amount of detail? transmission electron microscope 3.2 Stereomicroscope (Binocular Dissecting Microscope) (LM pages 22–23) Identifying the Parts (LM pages 22) 2. What is the magnification of your eyepieces? 10 5. Locate each of these parts on your stereomicroscope, and label them on Figure 3.3. Figure 3.4 (left, top to bottom): eyepiece lenses, binocular head; (right, top to bottom): magnification changing knob, illuminator, focusing knob Focusing the Stereomicroscope (LM pages 23) 4. Does your microscope have an independent focusing eyepiece? yes, most likely Is the image inverted? no 5. What kind of mechanism is on your microscope? Answers will vary. 6. and 7. The object will vary but only a portion of the object will be circled at the highest magnification. 3.3 Use of the Compound Light Microscope (LM pages 24–29) Identifying the Parts (LM pages 24–25) Identify the following parts on your microscope, and label them in Figure 3.4. Figure 3.4 (left, top to bottom): eyepiece(s) (ocular lens or lenses); body tube; nosepiece; objective lens or lenses; stage or mechanical stage (optional); diaphragm/diaphragm control lever; condenser Figure 3.4 (right, top to bottom): arm; stage clips; coarse-adjustment knob; fineadjustment knob; light source; base 1. What is the magnifying power of the ocular lenses on your microscope? The magnifying power of the ocular lens is marked on the lens barrel (usually 10). 5. a. What is the magnifying power of the scanning lens on your microscope? usually 4 14 b. What is the magnifying power of the low-power objective lens on your microscope? The magnifying power of the low-power objective lens is marked on the lens barrel (usually 10). c. What is the magnifying power of the high-power objective lens on your microscope? The magnifying power of the high-power objective lens is marked on the lens barrel (usually 40). d. Does your microscope have an oil immersion objective? depends on microscope 14. Does your microscope have a mechanical stage? depends on microscope Inversion (LM page 26) Observation: Inversion (LM page 26) 1. Draw the letter e as it appears on the slide (with the unaided eye, not looking through the eyepiece). The letter should be in the normal position. 2. Draw the letter e as it appears when you look through the eyepiece. The letter should be upside down and reversed. 3. What differences do you notice? The letter is inverted—that is, it appears to be upside down and backward compared to its appearance when viewed by the unaided eye. 4. Which way does the image appear to move? When moved to the right, the object appears to move to the left. 5. Which way did the image move. opposite direction Focusing the Microscope—Higher Powers (LM page 26) 4. On a drawing of the letter e, draw a circle around the portion of the letter that you are now seeing with high-power magnification. Portion will vary but a smaller portion is in view. Total Magnification (LM page 27) Observation: Total Magnification (LM page 27) Table 3.2 Total Magnification* Objective Ocular Lens Objective Lens Scanning power (if present) 10 4 Low power 10 10 High power 10 40 Oil immersion (if present) 10x 95-100x Total Magnification 40 100 400 O 950-1000x *Answers may vary with equipment. 3.4 Microscopic Observations (LM pages 27–30) Human Epithelial Cells (LM page 28) Observation: Human Epithelial Cells 3. Label Figure 3.6. 1. plasma membrane; 2. nucleus; 3. cytoplasm Onion Epidermal Cells (LM page 28) 15 Observation: Onion Epidermal Cells 4. Label Figure 3.7. 1. nucleus; 2. cell wall Table 3.3 Differences Differences Between Human Epithelial and Onion Epidermal Cells Shape Orientation Human Epithelial Cells (Cheek) Flattened, rounded Random orientation Boundary Thin Onion Epidermal Cells Square or rectangular Oriented end to end and in lines/rows Thick Euglena (LM page 29) Observation: Euglena (LM page 29) 5. How do your specimens compare with Figure 3.8? Answers will vary. LABORATORY REVIEW 3 (LM page 30) 1. Of the three types of microscopes studied, which one best shows the surface of an object? scanning electron microscope 2. Explain the designation “compound light” microscope: a. compound There are two sets of lenses—objective and ocular. b. light Light is used to view the object. 3. What function is performed by the diaphragm of a microscope? The diaphragm regulates the amount of light for viewing the object. 4. Briefly describe the necessary steps for observing a slide at low-power under the compound light microscope. Center the slide on the stage. Looking from the side, decrease the distance between the slide and the objective lens until the lens comes to a stop. Looking through the ocular lens(es), use the coarse-adjustment knob to increase the distance between the slide and the lens until the object comes into view. Adjust the light, and fine-adjust the focus. 5. Why is it helpful for a microscope to be parfocal? Little, if any, adjustment is needed when switching from low to high power. 6. Why is locating an object more difficult if you start with the high-power objective than with the low-power objective. The amount of the object you can see is smaller in high power than in low power. 7. How much larger than normal does an object appear with a low-power objective lens? 10X (ocular lens) X 10X objective lens = 100X 8. A virus is 50 nm in size. Would you recommend using a stereomicroscope, compound light microscope, or an electron microscope to see it? electron microscope Why? Only 16 an electron microscope has the capability of observing an object this small because it magnifies more and has greater resolving power. 9. What type of microscope, aside from the compound light microscope, might you use to observe the organisms found in pond water? stereomicroscope 10. State two differences between onion epidermal cells and human epithelial cells. Human epithelial cells are flat, round, and have a random orientation. Onion cells are square and oriented end to end in rows. 17 Laboratory 4 Cell Structure and Function (LM pages 31–44) Fourth Edition The prokaryotic cell was removed from this laboratory. Otherwise, this lab is essentially the same as it was in the previous edition. Special Requirements Living material. Elodea, living, for Section 4.1and 4.3; whole sheep blood for Section 4.3 Fresh material. Potato for Section 4.3. MATERIALS AND PREPARATIONS Instructions are grouped by procedure. Some materials may be used in more than one procedure. 4.1 Animal Cell and Plant Cell Structure (LM pages 32-35) ______ Elodea, living ______ forceps, dissecting fine point, stainless steel ______ dropping bottles ______ microscopes, compound light ______ lens paper ______ slides ______ coverslips Elodea (LM page 35,40). Live Elodea can be purchased locally at aquarium stores or a supply house. Place Elodea in distilled water in an aquarium with a continuous air supply from an aquarium air stone and pump. Place in indirect window light or under artificial illumination. 4.2 Diffusion (LM pages 36–37) Diffusion Through a Semisolid (LM page 36) ______ petri dish ______ gelatin powder or agar powder for 1.5% solution ______ potassium permanganate (KMnO4) crystals ______ wax pencils ______ rulers, plastic millimeter (preferably transparent) Diffusion demonstration through gelatin or agar (LM page 36). (Note: Agar allows faster diffusion than gelatin.) Prepare one dish per student group. At least a day ahead, prepare a 18 1.5% gelatin solution in a beaker or flask by dissolving 1.5 g of gelatin powder or agar in 100 ml of boiling water; stir thoroughly until dissolved. Allow to cool until the glassware can be handled with a hot mitt. Fill a petri dish 3 to 5 mm deep with gelatin solution. Put a lid on dish until cool. After cooling, store the dish in a refrigerator. After gelling, make a small depression in the center of the dish. Using forceps, drop a crystal of potassium permanganate into the depression. Potassium permanganate (LM page 36). Only 1 to 2 crystals are needed per student group. While wearing gloves, dispense several crystals of potassium permanganate into a shallow, wide-mouth, screw-top container appropriately labeled. (Note: Potassium permanganate diffuses very quickly.) Diffusion Across the Plasma Membrane (LM pages 36–37) ______ dialysis tubing, approximately 15 cm per setup ______ plastic droppers or Pasteur pipettes ______ rubber bands to close off the top of dialysis tubing ______ rubber bands that fit snugly around brim of 250 ml beaker ______ 1% glucose solution ______ 1–2% starch solution ______ beakers , 250 ml ______ water, distilled ______ iodine (IKI) solution ______ test tubes ______ test tube rack ______ wax pencils ______ Benedict’s reagent or glucose test strips, optional ______ boiling water bath: ______ hot plate (See Carolina’s Apparatus: Laboratory Equipment and Supplies section.) ______ large beaker ______ pair beaker tongs ______ test tube clamps ______ boiling chips, pumice ______ thermometer, Celsius 50–150°C range (See Carolina’s Apparatus: Thermometers section.) 1% glucose solution (LM page 37). This makes enough for all procedures for 20 student groups. Place 1 g of glucose in 50 mL of distilled water. Stir to dissolve, and bring the volume up to 100 mL. 1–2% starch solution (LM page 37). 20 mL per student group should be sufficient (using standard test tubes for all procedures). Care must be taken in preparing this solution. To make a 1% solution, dissolve 1 g of starch in a small amount of cold water to form a pourable paste. Add this to 100 mL of boiling distilled water, while stirring, and mix a few minutes. Cool. Add a pinch of sodium chloride (NaCl). If refrigerated, this solution will last for several weeks; otherwise, a fresh supply should be prepared each day. 19 Iodine (IKI) solution (LM page 37). Use one dropper bottle per student group. Pre-made iodine-potassium-iodide solution can be purchased, or the ingredients can be purchased separately as potassium iodide (KI) and iodine (I). These dry ingredients have a long shelf life Benedict’s reagent (LM page 37). 50 mL per student group is sufficient. Benedict’s reagent can be purchased as a powder to make 1 liter. Or to make 1 liter, mix 173 g of sodium citrate and 100 g of sodium carbonate, anhydrous (Na2CO3) (Carolina 88-8770) with 800 mL of distilled water. Warm this mixture to dissolve; then cool and filter it. Add distilled water to make 850 mL. Then dissolve 17.3 g of copper sulfate (cupric sulfate, pentahydrate) in 100 mL of distilled water, and stir slowly into the first solution. Add distilled water to make 1 liter. When testing, Benedict’s reagent should be boiled approximately 5 minutes or longer. Glucose test strips can be used in place of Benedict’s reagent to test for glucose in bag and beaker. Boiling water bath (LM page 37). Place a large beaker of water on a hot plate. Adjust the dial on the hot plate so that the water is maintained at a gentle rolling boil during the experiment. Thermometers are optional since students should know that boiling water is 100°C. 4.3 Osmosis: Diffusion of Water Across Plasma Membrane (LM pages 38–41) Experimental Procedure: Osmosis (LM pages 38–39) ______ Osmosis Demonstration Unit ______ 50% corn syrup solution ______ plastic syringe for filling thistle tube Osmosis Demonstration Alternative ______ dialysis tubing ______ beaker ______ 10–20% sucrose solution ______ rubber bands to close off the bottom of dialysis tubing ______ plastic clamps to close off the top of dialysis tubing Osmosis demonstration (LM page 38). The Osmosis Demonstration Unit is particularly easy to fill and empty. Partially fill the thistle tube with 50% corn syrup (or similar) solution. Place the apparatus in a beaker containing distilled water. Other osmometers can be found in Carolina’s Osmosis and Diffusion: Physiology section. Osmosis demonstration alternative (LM page 38). This demonstration can also be done using dialysis tubing and a beaker. See Experimental Procedure: Solute Diffusion Across the Plasma Membrane for setup. Tie off one end of the tubing, then fill with 10–20% sucrose solution. Clamp or tie it off at the open end. Pat the bag dry and weigh. Place the bag in a beaker of water for 45 minutes to 1 hour. Remove, pat dry, weigh immediately. Experimental Procedure: Demonstration of Tonicity in Red Blood Cells (LM pages 39-40) ______ test tubes, Pyrex 16 mm X 150 mm with stoppers ______ stoppers, rubber laboratory, solid, size 1 20 ______ ______ ______ ______ ______ ______ sheep blood, pooled, citrated water, distilled 0.8.% and 10% sodium chloride (NaCl) solutions dropping bottles, or bottles with droppers whole blood demonstration slides (optional) microscopes, compound light Whole blood (LM page 39). Blood should not be human blood. Use any available animal blood, other than human, to remove the risk of transmission of the HIV virus. Use caution with any animal blood as it may contain pathogens. Blood is shipped in iced, insulated containers and should be stored in the refrigerator. If kept refrigerated, sheep blood may be stored for up to 2 weeks.Prepare the test tubes as follows: Tube 1: 5 ml 0.8.% NaCl plus three drops of sheep blood Tube 2: 5 ml 10% NaCl plus three drops of sheep blood Tube 3: 5 ml 0.8.% NaCl plus distilled water and three drops of sheep blood. Stopper the tubes. To prepare the NaCl solutions: 0.8.% NaCl: Add 8. g of NaCl to 1 liter of distilled water. Smaller volumes may be prepared. 10% NaCl: Add 100 g of NaCl to 1 liter of distilled water. Smaller volumes may be prepared. Slides of whole blood (optional). Prepare a demonstration slide of the 0.8.% sheep blood solution (Tube 1) and the 10% sheep blood solution (Tube 2) for student observation. Experimental Procedure: Tonicity in Elodea Cells (LM pages 40) ______ See materials listed previously in Section 4.2. ______ 10% NaCl from the whole blood demonstrationExperimental Procedure: Experimental Procedure: Tonicity in Potato Strips (LM page 41) ______ potato, fresh ______ rulers, plastic millimeter ______ razor blades, single-edged ______ wax pencils ______ cutting board for potato ______ 10% sodium chloride (NaCl) in wash bottles ______ test tubes and racks ______ water ______ paper towels 4.4 pH and Cells (LM pages 42–43) Experimental Procedure: pH and Cells (LM page 42-43) ______ test tubes (3 per group) ______ test tube rack ______ pH 7 buffer (inorganic) solution ______ protein solution, buffered (e.g., albumin) 21 ______ ______ ______ ______ ______ ______ pH paper (range pH 0–14) stirring rods, glass 0.1 N hydrochloric acid (HCl) (see Carolina Chemicals, Hydrochloric Acid) 11.1 M conc. in plastic-coated safety bottle beakers, plastic 50 ml (two for each group) droppers water, distilled pH 7 buffer (LM page 42). 50 ml per student group is sufficient. If you wish to make it yourself, combine 50 ml 0.1 M potassium dihydrogen phosphate (KH2PO4), (1.36 g per 100 ml distilled water) with 28.1 ml 0.1 M NaOH (0.4 g per 100 ml distilled water). Dilute this mixture to 100 ml with distilled water. Buffered “cytoplasm” (e.g., albumin solution) (LM page 42). 50 ml per student group should be sufficient. Mix 1 g of albumin with 100 ml of pH 7.0 buffer. (Buffer may be purchased.) 0.1 N HCl solution (LM page 42). Mix 0.83 ml concentrated HCl with 100 ml distilled water. Place in dropper bottles. Experimental Procedure: Effectiveness of Antacids (LM page 43) ______ mortar and pestle ______ antacids: Alka-Seltzer, Rolaids, Tums, or other antacid tablet ______ 0.04% phenol red solution ______ beakers, plastic 250 ml ______ 0.1 N hydrochloric acid (HCl) (see Carolina Chemicals, Hydrochloric Acid) ______ rods, glass stirring ______ dropper EXERCISE QUESTIONS 4.1 Animal Cell and Plant Cell Structure (LM pages 32–43) Study Table 4.1 to determine structures that are unique to plant cells and unique to animal cells, and write them below the examples given: Plant Cells Animal Cells 1. Large central vacuole 1. Small vacuoles 2. Cell wall 2. Centriole 3. Chloroplast Animal Cell Structure (LM page 33) Label Figure 4.1 Answers follow. See Table 4.1 for a function of each labeled structure. 22 a. Nucleus i. Mitochondria b. Nuclear membrane j. Ribosome c. Nucleolus k. Smooth ER d. Cytoskeleton l. Lysosome e. Vesicle forming m. Cytoplasm f. Vesicle n. Plasma membrane g. Centrioles o. Golgi apparatus h. Rough ER Plant Cell Structure (LM page 34) Label Figure 4.2. See Table 4.1 for a function of these structures found in plant cells. a. Chloroplast h. Golgi apparatus b. Nucleus i. Mitochondrion c. Nucleolus j. Cytoplasm d. Rough ER k. Plasma membrane e. Ribosome l. Cell wall of adjacent cell f. Smooth ER m. Central vacuole g. Cytoskeleton n. Centrosome Observation: Plant Cell Structure (LM page 35) 6. Can you locate the cell nucleus? Answers will vary, but usually yes. 7. Why can't you see the other organelles featured in Figure 4.2. A light microscope does not permit seeing them; an electron microscope would be needed. 8. Can you detect the movement of chloroplasts in this cell or any other cell? Answers will vary, but usually yes. 4.2 Diffusion (LM pages 36–37) Experimental Procedure: Diffusion Through a Semisolid (LM page 36) 2. Length of time has been variable, dependent on when experiment began. 3. Measure (in mm) the movement of color from the center of the depression outward in one direction: Answers will vary. 4. Calculate the speed of diffusion mm/60min. Answers will vary. Calculate the speed of diffusion Answers will vary. 23 Table 4.2 Solute Diffusion Across Plasma Membrane At Start of Experiment Contents Color Bag Glucose Starch No color At End of Experiment Color Benedict’s Test Blue-black ____ Beaker Water Iodine Yellowish Less yellow Positive (+) Conclusion Iodine diffused into bag. Glucose diffused into bag. Conclusions: Solute Diffusion Across the Plasma Membrane (LM page 37) • Which solute did not diffuse across the dialysis membrane from the bag to the beaker? starch How do you know. The solution in the beaker did not turn blue-black. 4.3 Osmosis: Diffusion of Water Across Plasma Membrane (LM pages 38-39) Experimental Procedure: Osmosis (LM pages 38-39) 1. Note the level of liquid in the thistle tube, and measure how far it travels in 10 minutes: 1 mm 2. Calculate the speed of osmosis under these conditions: 6 mm/hr Conclusions: Osmosis (LM page 38) • In which direction was there a net movement of water? from beaker to thistle tube Explain what is meant by “net movement” after examining the arrows in Figure 4.6b. Water moves in and out of thistle tube, but more water moves in than moves out of tube. • If the starch molecules in corn syrup moved from the thistle tube to the beaker, would there have been a net movement of water into the thistle tube? no Why wouldn’t large starch molecules be able to move across the membrane from the thistle tube to the beaker? They are too large to cross a membrane. • Explain why the water level in the thistle tube rose: In terms of solvent concentration, water moved from the area of higher water concentration to the area of lower water concentration across a differentially permeable membrane. Tonicity in Cells (LM pages 39–40) Animal Cells (Red Blood Cells) page 39 Experimental Procedure: Demonstration of Tonicity in Red Blood Cells (LM pages 39–40) Table 4.3 Effect of Tonicity on Red Blood Cells Tube Tonicity Effect on Cells Print Visibility 1 Isotonic No effect No 2 Hypertonic Cells lose water No 3 Hypotonic Cells gain water Yes Explanation Cells are intact Cells are intact Cell have burst 24 Plant Cells (LM pages 40-41) Experimental Procedure: Tonicity in Elodea Cells (LM page 40) Table 4.4 Tonicity Hypotonic Hypertonic Effect of Tonicity in Elodea Cells Appearance of Cells Normal Shriveled center Due to (scientific term) Turgor pressure Plasmolysis Experimental Procedure: Tonicity in Potato Strips (LM page 41) 5. Which tube has the limp potato strip? tube 2 Use tonicity to explain why water diffused out of the potato strip in this tube.? The solution in tube 2 was hypertonic. Which tube has the stiff potato strip? tube 1 Use tonicity to explain why water diffused into the potato strip in this tube? The solution in tube 1 was hypotonic. 6. Use this space to create a table to display your results. Table Effect of Tonicity on Potato Strip Tube number Content Tonicity Results Explanation 1. water hypotonic stiff potato strip potato strip 2. salt solution hypertonic limp potato strip potato strip water diffused into potato strip water diffused out of potato strip Conclusion: Tonicity (page 41) In a hypotonic solution, animal cells swell to bursting. In red blood cells this is called hemolysis. In a hypertonic solution, animals cells shrivel. In red blood cells this is called crenation. In a hypotonic solution, the central vacole of Elodea cells exerts turgor pressure, and chloroplasts are seen next to the cell wall. In a hypertonic solution, the central vacuole loses water and plasmlysis occurs. The cytoplasm plus the chloroplast are seen in the center of the cell. In a hypotonic solution, potato strips gain water; in a hypertonic solution, potato strips lose water and become limp. 4.4 pH and Cells (LM pages 42–43) Why are cells and organisms buffered? to maintain pH of the cells Experimental Procedure: pH and Cells Table 4.5 Tube 1 2 3 * pH and Cells* Contents Water Buffer Cytoplasm pH Before Acid 6–6.5 7 7 pH After Acid 2–3 7 7 Explanation Not buffered Buffered Buffered These results are based on 1 ml of test solution. 25 Conclusions: pH and Cells (LM page 43) • Why would you expect cytoplasm to be as effective as the buffer in maintaining pH? Living things are buffered. Experimental Procedure: Effectiveness of Antacids (LM page 43) Table 4.6 Effectiveness of Antacids Data will depend on the antacids used. Conclusions: Effectiveness of Antacids (LM page 43) • Did dosage in mg have any effect on the results? depends on antacid used • Which of the substances on the label could be a buffer? depends on antacid LABORATORY REVIEW 4 (LM page 44) 1. What characteristics do all eukaryotic cells have in common? the presence of a nucleus and membrane-bounded organelles 2. Which organelle digests macromolecules and plant parts? lysosome 3. Why would you predict that an animal cell, but not a plant cell, might burst when placed in a hypotonic solution? Animal cells do not have cell walls. 4. Which of the cellular organelles would be included in the following categories: a. Membranous canals and vacuoles endoplasmic reticulum, Golgi apparatus, vesicles, vacuoles, lysosomes b. Energy-related organelles mitochondria and chloroplasts 5. How do you distinguish between rough endoplasmic reticulum and smooth endoplasmic reticulum? a. Structure Rough endoplasmic reticulum has ribosomes; smooth endoplasmic reticulum does not. b. Function Rough endoplasmic reticulum is the site of protein synthesis; smooth endoplasmic reticulum produces lipids. 6. If a dialysis bag filled with water is placed in a molasses solution, what do you predict will happen to the weight of the bag over time? The bag will lose weight. Why? Water would diffuse out of the bag and enter the molasses solution. 7. What is the relationship between plant cell structure and the ability of plants to stand upright? Strong cell walls and water-filled vacuoles that maintain turgor pressure help plants to stand upright.8. The police are trying to determine if material removed from the scene of a crime was plant matter. What would you suggest they look for? To determine if it was plant matter, the police should microscopically look for cell walls and chloroplasts, and they should test for starch. 26 9. A test tube contains red blood cells and a salt solution. When the tube is held up to a page, you cannot see the print. With reference to a concentration of 0.8.% sodium chloride (NaCl), how concentrated is the salt solution? The solution has a lower concentration than 0.9% NaCl since it is hypotonic to the cells and has caused them to burst. 10. Predict the microscopic appearance of cells in the leaf tissue of a wilted plant. The vacuole has pulled away from the cell wall, and the chloroplasts have moved to the center of the cell. 27 Laboratory 5 Enzymes (LM pages 45–52) Fourth Edition New to this edition, each section asks students to hypothesize the outcome of the experimental procedure before the experiment is done. In this way students can get a sense of the scientific process. MATERIALS AND PREPARATIONS Instructions are grouped by procedure. Some materials may be used in more than one procedure. Special Requirements Fresh material. A potato is needed per lab to prepare catalase for Sections 5.1-5.4. Equipment. Incubator (or water bath) and refrigerator (or ice bath) for 5.2 Effect of Temperature on Enzyme Activity. 15 minute incubation required. All Exercises _____ water, distilled _____ test tubes and racks _____ beakers _____ graduated transfer pipets 5.1 Catalase Activity (LM Pages 46–47) _____ catalase, buffered _____ hydrogen peroxide, purchased locally _____ 5% sucrose solution _____ potassium phosphate, dibasic _____ potassium phosphate, monobasic _____ pre-mixed buffer, pH7 Order solutions/agents or prepare your own. Buffered catalase (LM page 47). Make potato catalase fresh for each lab by grinding one small potato or half of a large potato with 50 mL water in a blender. Strain the potato mixture through a sieve to remove any large pieces of potato. Sections 5.1-5.3 requires buffered catalase. Section 5.4 requires nonbuffered catalase. You may put the buffered/nonbuffered catalase in a beaker on the supply bench, and students can use transfer pipettes to dispense the enzyme into test tubes. 28 Phosphate buffer (LM page 47). Add 7.70 g potassium phosphate, dibasic, K2HPO4, and 6.80 g potassium phosphate monobasic, KH2PO4, to one liter distilled water. Mix, check pH, and use to dilute catalase as needed. Premixed buffer may be used, as well. Hydrogen peroxide (LM page 47). The hydrogen peroxide used in this experiment can be purchased from a local store. 5% sucrose solution (LM page 47). Dissolve 5 g sucrose in 100 mL distilled water. Dispense from a beaker with dropper pipettes. Note: Caution the students that they should swirl the enzyme and substrate to mix, then allow the tube to sit for 20 seconds before measuring the height of the bubble column. The bubbles produced by the reaction are very small, and resemble shaving cream foam. If the catalase/sucrose mixture is swirled for 20 seconds, the catalase will produce large bubbles, which some students confuse for the enzyme reaction. 5.2 Effect of Temperature on Enzyme Activity (LM pages 47-48) _____ catalase (see section 5.1) _____ hydrogen peroxide (purchased locally) _____ incubator _____ refrigerator or ice bath _____ boiling water bath: _____ hot plate _____ large beakers _____ beaker tongs _____ thermometer _____ test tube holders 5.3 Effect of Concentration on Enzyme Activity (LM page 49) _____ catalase (see section 5.1) _____ hydrogen peroxide (purchased locally) 5.4 Effect of pH on Enzyme Activity (LM pages 50–51) _____ catalase, nonbuffered (see section 5.1) _____ 5 M HCl _____ hydrogen peroxide (purchased locally) _____ 5 M NaOH Order solutions/agents or prepare your own. 5 M HCl. CAUTION—This solution will get HOT (LM page 50). Add 400 mL distilled water to a 1-liter graduated beaker. Place beaker with magnetic spinbar on a stirring plate. While stirring, slowly pour in 416 mL concentrated HCl. Add distilled water to bring the volume up to 1,000 mL. 5 M NaOH. CAUTION—This solution will get very HOT (LM page 50). In a 1-liter beaker with a magnetic spinbar, gradually add a total of 200 grams NaOH pellets to 750 mL distilled water, allowing the heat to dissipate between additions of NaOH. After the solution cools, add distilled water to bring the volume up to 1,000 mL. 29 EXERCISE QUESTIONS 5.1 Catalase Activity (LM pages 46-47) What is the reactant in this reaction? H2O2 What is the substrate for catalase? H2O2 What are the products in this reaction? H2O and O2 Bubbling occurs as the reaction proceeds. Why? O2 production Hypothesize which tube 1, 2, or 3 in Table 5.1 will have the greater bubble column height. Include an explanation in your hypothesis. Tube 1 because only this tube contains both catalase and its substrate. Experimental Procedure: Catalase Activity (LM page 47) Table 5.1 Catalase Activity Tube Contents Bubble Column Height 1 Catalase 20 mm Hydrogen peroxide 2 Water 0 mm Hydrogen peroxide 3 Catalase 0 mm Sucrose solution Explanation Substrate and enzyme are both present. Tube lacks enzyme. Tube lacks correct substrate. Conclusions: Catalase Activity (LM page 47) • Which tube showed the bubbling you expected? tube 1. • Which tube is a negative control? tube 2 If this tube showed bubbling, what could you conclude about your procedure? Results are not due to catalase; therefore, experiment is invalid. • Enzymes are specific. Which tube exemplifies this characteristic of an enzyme? tube 3. 5.2 Effect of Temperature on Enzyme Activity (LM pages 47-48) With this information in mind, examine Table 5.2 and hypothesize which tube (1, 2, or 3) will have more product per unit time as judged by bubble height. tube 2 Include a complete explanation in your hypothesis. The enzyme exposed to normal body temperature will perform best because the molecules will be moving about but the temperature is not high enough to denature the enzyme. Experimental Procedure: Effect of Temperature (LM pages 48) 5. Record your results in Table 5.2. Plot your results in Figure 5.3. Table 5.2 Effect of Temperature Tube Temperature (°C) 1 Refrigerator 2 Incubator 5°C 37°C Bubble Column Height (mm) 8. mm 23 mm 3 Boiling water 100°C 0 mm Explanation Temperature too hot. Denaturation occurred. Temperature below optimum. Optimum temperature Conclusions: Effect of Temperature (LM page 48) 30 • Was your hypothesis supported. Depends on hypothesis. • What is your conclusion concerning the effect of temperature on enzyme activity? A warm temperature speeds an enzymatic reaction, but a hot temperature denatures an enzyme. 5.3 Effect of Concentration on Enzyme Activity (LM page 49) With this in mind, examine Table 5.3 and hypothesize which tube (1, 2, or 3) will have more product per unit time as judged by bubble column height. tube 3 Explain your answer. The more enzyme molecules, the more active sites per substrate, and the more product within a limited time frame. Experimental Procedure: Effect of Enzyme Concentration (LM page 49) Table 5.3 Effect of Enzyme Concentration Tube Amount of Bubble Column Explanation Enzyme Height (mm) 1 none 20 mm Explanation for all tubes: The 2 1 cm 30 mm greater the enzyme concentration, 3 3 cm 40 mm the more O2 during the allotted time Conclusions: Effect of Concentration (LM page 49) • Was your hypothesis supported. Depends on hypothesis. • If unlimited time was allotted, would the final results be the same in all tubes? Yes. Explain why or why not. All tubes have the same amount of substrate and enzymes can be used over and over again. • Would you expect similar results if the substrate concentration were varied in the same manner as the enzyme concentration? yes Why or why not? It would take less time for the substrate to encounter an active site. • What is your conclusion concerning the effect of concentration on enzyme activity? Increased amount of enzyme or substrate will increase the rate of enzyme activity. 5.4 Effect of pH on Enzyme Activity (LM page 50-51) With this information about catalase in mind, examine Table 5.4 and hypothesize which tube (1, 2, or 3) will have more product per unit time as judged by bubble height. tube 2 Include a complete explanation in your hypothesis. pH 7 is optimum pH for catalase Experimental Procedure: Effect of pH 1-3. Measure the height of the bubble column and record your results in Table 5.4. Plot your results from Table 5.4 here (Fig. 5.4). Table 5.4 Effect of pH Tube pH Bubble Column Height (mm) 1 3 17 mm 2 7 35 mm 3 11 12 mm Explanation pH too acidic for catalase optimum pH for catalase pH too basic for catalase Conclusions: Effect of pH (LM page 51) 31 • Was your hypothesis supported. depends on hypothesis. • What is your conclusion concerning the effect of pH on enzyme activity? Any pH other than the optimum pH will decrease the activity of an enzyme. 5.5 Factors that Affect Enzyme Activity (LM page 51) Table 5.5 Factors that Affect Enzyme Activity Factors Promote Enzyme Activity Enzyme Specificity Active site available Temperature Intermediate Enzyme or substrate High concentration pH Optimum pH Inhibit Enzyme Activity Active site not available Extreme Low Too acidic or basic for the enzyme Conclusions: Factors that Affect Enzyme Activity (LM page 51) • Why does enzyme specificity promote enzyme activity? The active site of an enzyme has a shape that is complementary to the shape of its substrate. In this way the enzyme brings together the substrates so that the reaction occurs. • Why does a warm temperature promote enzyme activity? It increases the motion of molecules and therefore the number of times substrates find the active site. • Why does increasing enzyme concentration promote enzyme activity? It increases the number of active sites available. • Why does optimum pH promote enzyme activity? Optimum pH is required to maintain the shape of the active site of an enzyme. LABORATORY REVIEW 5 (LM page 52) 1. What happens at the active site of an enzyme? Substrates are oriented to bring about the reaction.2. On the basis of the active site, explain why the following conditions speed a chemical reaction: a. More enzyme There are more active sites available for substrates. b. More substrate It is more likely that a substrate molecule will encounter an active site. 3. Name two other conditions (other than the ones mentioned in question 2) that maximize enzymatic reactions. a. optimum temperature b. optimum pH 4. Explain the necessity for each of the two conditions you listed in question 3. a. Movement of molecules increase as temperature rises b. Enzyme shape is maintained 5. Lipase is a digestive enzyme that digests fat droplets in the basic conditions (NaHCO3 is present) of the small intestine. Indicate which of the following test tubes would show digestion following incubation at 37°C, and explain why the 32 others would not. Tube 1: Water, fat droplets No enzyme Tube 2: Water, fat droplets, lipase Wrong pH Tube 3: Water, fat droplets, lipase, NaHCO3 Digestion occurs Tube 4: Water, lipase, NaHCO3 No substrate 6. Fats are digested to fatty acids and glycerol. As the reaction described in question proceeds, the solution will become what type pH? acidic Why? Fatty acids are present. 7. Given the following reaction: catalase 2 H2O2 H2O + O2 hydrogen water oxygen peroxide a. Which substance is the substrate? hydrogen peroxide b. Which substance is the enzyme? catalase c. Which substances are the end products? water and oxygen d. Is this a synthetic or degradative reaction? degradative How do you know? The larger molecule on the left becomes the two smaller molecules on the right. 33 Laboratory 6 Photosynthesis (LM pages 53–62) Fourth Edition This lab remains the same as it was in the previous edition. MATERIALS AND PREPARATIONS Instructions are grouped by exercise. Some materials may be used in more than one exercise. Special Requirements Fresh material. Fresh or frozen spinach, depending on preparation alternative chosen, for 6.1 Photosynthetic Pigments Living material. Duckweed or Elodea (Anacharis) for 6.2 Solar Energy and 6.3 Carbon Dioxide Uptake Equipment, preassembly required. Volumeter for 6.2 Solar Energy and 6.3 Carbon Dioxide Uptake Fume hood for 6.1 Photosynthetic Pigments All Exercises _____ safety goggles (See Carolina’s Safety: Face Protection Section) _____ latex gloves and/or nonlatex gloves (See Carolina’s Safety: Hand Protection Section) _____ lab coats (See Carolina’s Safety: Body Protection Section) or other clothing protection _____ distilled water _____ wax pencils _____ rulers, plastic centimeter 6.1 Plant Pigments (LM pages 54–55) _____ fresh spinach pigment extract: _____ spinach, fresh _____ blender, glass or stainless steel _____ cheesecloth _____ polypropylene utility funnel, 4 ¼”, or Buchner funnel _____ filter paper _____ acetone _____ frozen spinach pigment extract alternative: _____ spinach, frozen, 40 g _____ blender, glass or stainless steel _____ acetone 34 _____ _____ _____ _____ _____ _____ _____ _____ _____ _____ _____ ethanol _____ sodium chloride (Crystal) _____ filter, paper _____ amber bottle(s) test tubes, rimless, large culture 25 × 150 mm cork with paper-clip hook for large test tubes chromatography paper, Whatman #1 glass capillary tube paper towels scissors fume hood chromatography solution: _____ petroleum ether _____ acetone _____ jar, wide-mouth, screw-cap test tube rack, 25 mm holes pencils Whatman #1 chromatography paper (LM page 54). Use sheets of 12 × 12 cm Whatman #1 chromatography paper. Cut the sheets to fit the chromatography apparatus, rounding or pointing one end. Fume hood and cautions (LM page 54). For the chromatography exercise, direct students’ attention to the fume hood and ether cautions in the Lab Manual. Fresh spinach pigment extract (LM page 55). If a fume hood is available, prepare the extract there. Wash and thoroughly drain the spinach. Cut the veins and petioles from the leaves. Put the spinach in a glass or stainless steel blender, add acetone, and blend to form a thick slurry. Extract should be filtered, using a cheesecloth plug in a funnel or a small Buchner funnel with aspiration. Refrigerate the slurry in a tightly stoppered container labeled “Pigment Extract.” Extract exposed to light and room temperature begins decomposing within an hour, while refrigerated extract may last overnight. An alternate method involves drying spinach leaves slowly in a dry oven and then pulverizing them in a blender or with a mortar and pestle. Leaf powder is useful for weeks if stored in a sealed container and placed in a cool, dark area. Pulverization reduces leaf volume considerably. The dry leaf powder can be added to a small amount of acetone to form a thick slurry. Frozen spinach pigment extract alternative (LM page 55). Partially defrost and divide a package of frozen spinach into 40 g portions. Combine 40 g frozen spinach with 200 ml acetone in a blender. Blend 2 to 3 minutes on high. Let stand 3 minutes. Decant supernatant, save as 1. Add 100 ml ethanol to solids remaining in blender. Blend 2 to 3 minutes on high. Decant supernatant, save as 2. Combine 1 and 2, and filter to remove any remaining solids. Add a pinch of sodium chloride. Refrigerate in amber jar. Chromatography solution (LM page 55). 100 ml is sufficient for five student groups. Combine forty-five parts petroleum ether with five parts acetone, and store in a screw-capped 35 container. Label as “Chromatography Solution.” Keep the container tightly closed, since this solution is volatile and extremely flammable. (If a fume hood is available, prepare the solution there.) Have a wide-mouth, screw-capped jar, labeled “Used Chromatography Solution,’’ available in which to place used solution. Keep the jar tightly closed. Disposal (LM page 55) Organic solvents should be recycled or disposed of according to local procedures and regulations. 6.2 Solar Energy (LM pages 56–59) _____ Duckweed or Elodea, fresh _____ aeration equipment for Elodea, aquarium air pump 10-20 gal tank _____ razor blades, single-edged _____ volumeter: _____ test tubes, large culture, 25 × 150 mm rimless _____ rubber stoppers, #5, single-holed _____ glycerol _____ pipet, graduated _____ sodium bicarbonate powder 3% (NaHCO3) solution _____ water, distilled _____ aquarium aerator for sodium bicarbonate _____ test tube rack for 25 mm tubes _____ beaker, 1,000 ml (plastic, glass) _____ lamp, 150 watt or aquarium light (full-spectrum bulb) Volumeter (LM page 57). Prepare one volumeter per student group ahead of time. Insert a graduated pipette into a single-holed rubber stopper that fits into a large culture test tube, as shown in Figure 6.4. When the rubber stopper is in place during the experiments, a continuous column of liquid will form between the test tube and the pipette. Adjust the placement of the leading edge of the liquid by applying pressure to the stopper. The oxygen emitted by the Elodea will displace the liquid in the test tube, thus moving the edge of the liquid in the pipette. The student will read the change in millimeters. 3% sodium bicarbonate (NaHCO3) solution (LM page 56) Prepare 125 ml per student group. Dissolve 30 g of NaHCO3 in 1,000 ml of distilled water. Aerate the solution with an aquarium aerator for 30 minutes before the laboratory exercise to saturate with carbon dioxide. Discard the solution after use. Elodea (LM page 56) Use fresh duckweed or Elodea (one healthy sprig per student group is sufficient) that has been maintained in continuously aerated distilled water. Change the water at least every two days. 6.3 Carbon Dioxide Uptake (LM page 60) _____ 0.04% phenol red solution _____ straws, individually packaged _____ Elodea (or duckweed), _____ Volumeter as described in Section 7.2. 36 0.04% phenol red solution (LM page 60). Prepare 100 ml per student group. Dissolve 0.04 g of phenol red in 100 ml of distilled water. Have students use caution when blowing through the straw into the test tube of phenol red. Overzealous students may blow the phenol red out of the tubes and onto themselves. Students need only blow on the surface of the liquid to get a color change. EXERCISE QUESTIONS 6.1 Plant Pigments (LM pages 54–55) Experimental Procedure: Plant Pigments (LM page 54–55) 9. Which pigment is the most nonpolar (that is, has the greatest affinity for the nonpolar solvent)? beta-carotene 10. Calculate the Rf (ratio-factor) values for each pigment. Beta-carotenes will have the largest values, which will be less than one, and chlorophyll b will have the smallest. 11. Do your results suggest that the chemical characteristics of these pigments might differ? Yes Why? They must differ, otherwise all the pigments would migrate the same distance. 6.2 Solar Energy (LM pages 56–59) Verify that photosynthesis releases oxygen by writing the equation for photosynthesis. solar energy CO2 + H2O —————————————> (CH2O)n + O2 Role of White Light Experimental Procedure: White Light (LM pages 56–58) 4.Measure in millimeters the distance the edge moved. Why did the edge move forward? The edge moved in response to oxygen production, which forced the liquid outward in the tubing. 5.Record the length of time it takes for the edge of the solution in the tubing to recede 1mm. Answers cab vary. Why does cellular respiration, which occurs in a plant all the time, cause the edge to recede? Oxygen, which was produced during photosynthesis, was being used by the plant during cellular respiration. As the volume of oxygen decreased (because photosynthesis is not occurring when the tube is wrapped by foil), less water was forced into the tubing, and the edge receded. 6. If the Elodea had not been respiring in step 4, how far would the edge have moved? Add the distance the edge receded to the distance the edge moved forward during the initial experiment with the white light. 7. Calculate the rate of photosynthesis. 201 mm/hr (Rates will vary with plant condition, distance from the lamp, and room temperature.) Table 6.2 Rate of Photosynthesis (White Light) Net photosynthesis (white light) Cellular respiration (no light) Gross photosynthesis (net + cellular respiration) Rate of photosynthesis Data 32/10 min 1.5/10 min 33.5 (mm/10 min) 201 (mm/hr) 37 Role of Green Light According to Fig. 6.5, what color light do the chlorophylls absorb best? violet, blue, and red Least? green, the reflected color What color light do the carotenoids (carotenes and xanthophylls) absorb best? greenyellow Least? yellow, orange, the reflected colors Does photosynthesis use green light? not extensively Experimental Procedure: Green Light (LM pages 58–59) 8. Based on your data in Table 6.3, this percentage = 37% Based on class data in Table 6.3, this percentage = Compute from class data. Table 6.3 Rate of Photosynthesis (Green Light) Your Data Class Data Gross Photosynthesis (mm/10 min) White (from Table 6.2) 33.5 mm/10 min Green 11.5 mm/10 min Rate of Photosynthesis (mm/hr) White (from Table 6.2) 201 mm/hr Green 75 mm/hr Note: The results presented in this table are sample data. Actual results will vary. Conclusions (LM page 59) • Explain why the rate of photosynthesis with green light is only a portion of the rate of photosynthesis with white light. Photosynthesis does not use green light extensively. • How does the percentage based on your data differ from that based on class data? Explanation will vary according to particular student. 6.3 Carbon Dioxide Uptake (LM page 60) Experimental Procedure: Carbon Dioxide Uptake (LM page 64) 2. Blowing onto the solution adds what gas to the test tube? primarily carbon dioxide When carbon dioxide combines with water, it forms carbonic acid. What causes the color change? Carbonic acid releases hydrogen ions. As the pH decreases, the color of the indicator changes from red to yellow. 6. Hypothesize why the solution in the test tube eventually turned red. The plant uses carbon dioxide in photosynthesis. As carbon dioxide is absorbed, carbonic acid is reconverted to carbon dioxide and water. When the plant has taken up all the blown-in carbon dioxide, the amount of hydrogen ions and, therefore, the pH of the solution, returns to the previous level. Therefore, the phenol red returns to its initial color. Use of a Control (LM page 60) • Considering the test sample in Table 6.4, suggest a possible control sample for this experiment: a sample that does not contain Elodea but that contains phenol red with carbon dioxide blown in to produce the same yellow color • Why should all experiments have a control? In a control sample, the variable 38 being tested is missing. Therefore, if a control sample gives positive results, the experiment is invalid—the reagents may be contaminated or the procedure may need improvement. Table 6.4 Carbon Dioxide Uptake Tube Time for Color Change Test sample: Elodea + phenol red solution + CO2 30–40 minutes Control sample: CO2 + phenol red solution No change 6.4 Carbon Cycle (LM page 61) 1. Which organelle in plants carries out the reaction in the previous equation in the reverse (right-to-left) direction? chloroplast 2. Pertaining to photosynthesis, the energy in the equation is provided by solar energy. 3. Which organelle in plants and animals is involved in carrying out the reaction in this equation in the forward direction? mitochondria 4. Pertaining to cellular respiration, the energy in the equation becomes chemical bond energy in what molecule? ATP 5. Would it be correct to say that solar energy eventually becomes the chemical bond energy in ATP? yes Why? Because solar energy becomes chemical bond energy of carbohydrates and chemical bond energy of carbohydrates becomes that of ATP molecules. 6. Considering that both plants and animals carry on cellular respiration, revise Figure 6.6 to improve its accuracy. The corn plants should be placed next to the cow also. LABORATORY REVIEW 6 (LM page 62) 1. Where In do the light reactions of photosynthesis take place? In thylakoid membranes of chloroplasts. 2. What procedure did you use to separate plant pigments? chromatography 3. What determines the speed with which a pigment moves up the chromatography paper? solubility in a solvent 4. Where do plants ordinarily get the energy they need to carry on photosynthesis? white light of solar energy 5. Blue, red, and green light are all present in what color of light? white 6. Why do blue and red light, but not green promote photosynthesis. Chlorophyll is able to absorb blue and red light but not green light. 7. Does Elodea respire in the light or in the dark? Both the light and the dark 8. Phenol red turns what color when carbon dioxide is added? yellow 9. What happens to carbon dioxide during photosynthesis? It is converted to carbohydrate. 39 10. Some plants are colorless. Do you predict that they carry on photosynthesis. No, a plant requires a pigment to absorb solar energy and photosynthesize. 40 7 Cellular Reproduction (LM pages 63-72) Fourth Edition This edition has a separate lab for mitosis and another for meiosis. The mitosis lab gives students an exercise to do as they learn the phases of mitosis. MATERIALS AND PREPARATIONS Instructions are grouped by exercise. Some materials may be used in more than one exercise. All Exercises ________microscopes, compound light ________lens paper 7.2 Animal Cell Mitosis and Cytokinesis Animal Mitosis (LM page 66-68) ______ mitosis models, animal ______ slide, prepared: whitefish mitosis 7.3 Plant Cell Mitosis and Cytokinesis Plant Mitosis (LM page 68-71) ______ mitosis models, plant ( ______ slide, prepared: onion (Allium) root tip Note: For the experimental procedure regarding time span of phases, it would be best to have students pool their data; a minimum of 40 observed cells should work well. EXERCISE QUESTIONS 7.1 The Cell Cycle (LM pages 64–65) State the event of each stage on the line provided. G1 Organelles begin to double in number. S Replication of DNA G2 Synthesis of proteins M Mitosis Explain why the entire process is called the “cell cycle.” In dividing cells, the stages repeat. The S Stage of the Cell Cycle (LM page 65) Label the sister chromatids, and centromere in the drawing of a duplicated chromosome in Figure 7.3 1. sister chromatids; 2. centromere 41 Each daughter cell will have 18 chromosomes. The M Stage of the Cell Cycle (LM page 65) Consult Figure 7.2 and write the phases of mitosis here: prophase, metaphase, anaphase, telophase 7.2 Animal Cell Mitosis and Cytokinesis (LM page 68) Observation: Animal Cell Mitosis (LM page 66) 1. What is the number of chromosomes in the parent cell and in the daughter cells in this model series? Answer may vary, depending on what model is being used. 3. Do these models show the spindle, which is illustrated in Figure 7.4? Most likely yes. 4. What is the shape of animal cells? Blastula cells are round. What is the appearance of the spindle pole? An aster is present. Whitefish Blastula Slide (LM page 67) 3. Match these statements to the correct phase of animal cell mitosis to Figure 7.5 and write the correct statements on the lines provided. Prophase: Duplicated chromosomes have no particular arrangement in the cell Metaphase: Duplicated chromosomes are aligned in the equator of the spindle Anaphase: Daughter chromosomes are moving to the poles of the spindle Telophase: Two daughter cells are now forming 4. Explain the different appearance of the chromosomes. In the prophase cell, the chromosomes are duplicated and in the telophase cell, the chromosomes are single. Cytokinesis in Animal Cells (LM page 68) Are any of the cells in your whitefish blastula slide undergoing cytokinesis? Most likely yes. Do you see any cleavage furrows? Most likely yes. 7.3 Plant Cell Mitosis and Cytokinesis (LM page 68-71) Observation: Plant Cell Mitosis (LM page 69) 3. What is the number of chromosomes in each of the cells in this model series? Answer may vary, depending on what model is being used. Onion Root Tip Slide (LM page 69) Time Span for Phases of the Cell Cycle in the Onion Root Tip (LM p. 69-70) Hypothesize how many minutes the cell spends during each of these phases of the cell cycle. Hypotheses will most likely vary. Experimental Procedure Time Span for Phases of the Cell Cycle in the Onion Root Tip (LM p. 70) 3. Calculate the percentage of cells in each phase of the cell cycle and record in Table 7.2. 4. Calculate the time span for each phase of the cell cycle and record in Table 7.2. 42 Table 7.2 Time Span for Phases of the Cell Cycle in the Onion Root Tip Phase Number Seen % of Total Time Span (min) Interphase 30 75 1080 Prophase 4 10 144 Metaphase 1 2.5 36 Anaphase 2 5 72 Telophase 3 7.5 108 Total 40 100 1440 Conclusions: Time Span for Phases of the Cell Cycle in the Onion Root Tip (LM 70) Were your hypotheses supported or not supported by your observation of onion root tip cells undergoing the cell cycle? Answers will vary. Suggest a possible explanation for the length of time a cell spends on different phases of the cell cycle. During interphase a cell is carrying on normal activities; During prophase, a cell gets ready for metaphase because the chromosomes must be positioned correctly and this allows the daughter chromosomes to quickly separate during anaphase. Telophase takes longer because the cells reorganize into daughter cells. Cytokinesis in Plant Cells (LM page 71) Were any of the cells undergoing cytokinesis as shown in Figure 7.7 during telophase? Most likely, yes. How do you know? Cell plate is present. Offer an explanation for why Figure 7.8 is so detailed. It’s an electron micrograph. Would you predict that the vesicles of the cell plate lay down the new cell wall inside or outside the vesicles? Explain your answer. Inside, because this is where the components to make the cell wall accumulate. LABORATORY REVIEW 7 (LM page 72) 1. Divide the cell cycle into two main portions, and tell in general what is happening in these two portions. Interphase (cells are contributing to the workings of the body) and Mitosis (cells are dividing). 2. Most of the time the cell is in which of these portions of the cell cycle? Why is this advantageous? Interphase because this is the time that cells go about their normal activities. 3. Name and define the two events that take place when a cell divides. Mitosis (nuclear division) and cytokinesis (division of cytoplasm) occur when a cell divides. 4. What is the function of the centromere during mitosis? The centromere provides a place of attachment for spindle fibers. 43 5. Evolution of the spindle was of central importance to eukaryotic cells. What role is played by the spindle during mitosis? The chromosomes move because they are attached to spindle fibers that lengthen (push) and shorten (pull) the chromosomes. 6. Do the chromosomes have sister chromatids during these phases of mitosis (yes or no)? a. Prophase Yes b. Metaphase Yes c. Anaphase No d. Telophase No 7. Contrast the appearance of animal cells and plants during mitosis. Animal cells have asters but plant cells do not have asters. 8. Explain how it is possible for each phase of mitosis and the daughter cells to have the same number of chromosomes. Duplication of chromatids provides the daughter nuclei for daughter cells. Counting the centromeres tells the number of chromosomes. 9. Contrast how cytokinesis occurs in animal cells with how it occurs in plant cells. Cytokinesis in animal cells occurs by furrowing; in plant cells it occurs by formation of a cell plate. 10. What would a tissue look like if cytokinesis did not occur? The tissue would have multiple nuclei. 44 Laboratory 8 Sexual Reproduction (LM pages 73–82) Fourth Edition This edition has a separate lab for mitosis and another for meiosis. Section 8.2 now emphasizes how meiosis results in variation among the gametes. Text art was added to this section as a guide to the hands-on experimental procedure in this section. MATERIALS AND PREPARATIONS Instructions are grouped by exercise. Some materials may be used in more than one exercise. 8.1 Meiosis: Reduction Division (LM pages 74-75) Meiosis in Lily Anther (p. 74) ______ Microscope slide of meiosis in Lily anther 8.2 Production of Variation During Meiosis Building Chromosomes to Simulate Meiosis (LM page 76-78) ______ Chromosome Simulation ______ scissors ______ Animal meiosis model74 8.3 Human Life Cycle ______ Paper and pencil EXERCISE QUESTIONS 8.1 Meiosis: Reduction Division (LM pages 74-75) Before proceeding a. Distinguish between diploid (2n ) and haploid (n): Diploid equals the full number of chromosomes when there are pairs of chromosomes. Haploid equals half the full number of chromosomes when each type chromosome does not have a pair. b. Distinguish between a homologue and a tetrad: Each member of a pair of chromosomes is called a homologue. Tetrads occur when the homologues are paired and each one consists of two chromotids; therefore four chromatids are in close proximity. Observation: Meiosis in Lily Anther (LM pages (74-75) Phases of Meiosis I (LM page 74-75) 45 2. Tell what is happening in each of these (meiosis I) phases. See descriptions in Figure 8.3 Meiosis I 3. In Figure 8.3, place a 2n or n beside each drawing. Cells in prophase I, metaphase I, and anaphase I are 2n; each cell forming in Telophase I and daughter cells in interkinesis are n. Phases of Meiosis II (LM page 75) 2. Tell what is happening in each of these (meiosis II) phases. See descriptions in Figure 8.4 Meiosis II. 3. In Figure 8.4, place a 2n or n beside each drawing. All cells during meiosis II are n. 8.2 Production of Variation During Meiosis (LM 76-78) Anaphase II (LM page 78) 10. Pull the two magnets of each duplicated chromosome apart. What does this action represent? This action represents separation of centromeres and daughter chromosomes moving to opposite poles. Telophase II (LM page 78) 11. At the end of telophase, the daughter nuclei reform. • Therefore, how many nuclei are usually present when meiosis II is complete? four • In this exercise, how many chromosomes were in the parent cell nucleus undergoing meiosis II? two • How many chromosomes are in the daughter nuclei? two Explain. When the chromatids of the chromosomes in the parental cell separate, they become daughter chromosomes, which go into the daughter cells. Summary of Production of Variation During Meiosis (LM page 78) 1. If the parent cell is 2n=4, the daughter cells are n = 2. 2. Why do the puppies born to these parents show variation? a. This process is called crossing-over. b. During metaphase I, align independently, and therefore differently. This means that daughter cells following telophase I can have different combinations of chromosomes. c. During fertilization, variant sperm fertilize variant eggs helping to ensure that the new individual inherits a different combination of chromosomes than the parent had. 8.3 Human Life Cycle (LM pages 79-81) As you read the following text, fill in boxes in Figure 8.6 with the terms “mitosis” or “meiosis.” Left to right: meiosis, mitosis, mitosis. Summary of Human Life Cycle (LM page 79) Fill in the blanks to ensure your understanding of the role of meiosis and mitosis in humans. 1. Name of organ that produces gametes in males testes; in females ovaries 46 2. Name of process that produces gametes in males spermatogenesis; in females oogenesis 3. Type of cell division involved in process in males meiosis; in females meiosis 4. Name of gamete in males sperm; in females egg 5. Number of chromosomes in gamete in males n; in females n 6. Results of fertilization zygote 7. Number of chromosomes provided 2n Mitosis Versus Meiosis (LM pages 80) Table 8.1 Differences Between Mitosis and Meiosis 1. Number of divisions 2. Chromosome number in daughter cells 3. Number of daughter cells Table 8.2 Mitosis One Same as the parent cell Two Meiosis Two One-half of the parent cell Four Mitosis Compared with Meiosis I Mitosis Prophase: No pairing of chromosomes Metaphase: Duplicated chromosomes at metaphase plate Anaphase: Sister chromatids separate Telophase: Chromosomes have one chromatid Meiosis I Prophase I: Pairing of homologues Metaphase I: Duplicated homologues at equator Anaphase I: Homologues separate Telophase I: Chromosomes have two chromatids. Provide the correct term for each definition. (LM page 80) 1. Type of cell division that keeps the chromosome number the same and occurs during growth and repair Mitosis 2. Type of cell division that reduces the chromosome number and occurs during gamete formation Meiosis 3. Half the diploid number of chromosomes Haploid (n) number 4. Male gamete with the n number of chromosomes Sperm 5. Female gamete with the n number of chromosomes Egg LABORATORY REVIEW 8 (LM page 82) 1. Where would you expect to find meiosis taking place in human males? testes females? ovaries 47 2. If there are 13 pairs of homologues in a parent cell, how many chromosomes are there in a sperm? 13 Explain how you arrived at this number. Pairs separate during meiosis. 3. Account for why each of your body cells contains two of each kind of chromosome. Prior to mitosis, DNA replicated and the chromosomes duplicated. Separation of chromatids provides the 2n number for each cell. 4. Does metaphase of mitosis, meiosis I, or meiosis II have the haploid number of chromosomes at the equator of the spindle? meiosis II 5. List four differences when comparing mitosis with meiosis. Figure 8.7 See Table 8.2 and 6. If the cells of an organism have 12 chromosomes, what is the number of chromosomes at the equator during metaphase of mitosis? 12 during metaphase of meiosis II? 6 7. A student is simulating meiosis I with a pair of homologues that are red-long and yellow-long. Why would you not expect to find both red-long and yellow-long in one resulting daughter cell? Red-long and yellow-long are homologues and homologues separate during meiosis. 8. With reference to a pair of homologues, describe the change in the two participating nonsister chromatids following crossing-over. Each nonsister chromatid will have genetic material from the other nonsister chromatid. 9. What would be the appearance of a cell that completes mitosis but does not undergo cytokinesis? The cell would have two nuclei. with a cell that completes meiosis but does not undergo cytokinesis? The cell would have four nuclei. 10. In the life cycle of humans, when does mitosis occur? During growth and development of zygote and during growth of individual. 48 Laboratory 9 Patterns of Inheritance (LM pages 83–96) Fourth Edition This lab was rewritten to facilitate its use by the instructor because students observe only the results of live genetic crosses rather than performing the crosses. More paper and pencil practice is provided for students as they fill in Punnett squares and do additional genetics problems. New or Revised Figures: 9.2 What are the expected results of a cross?; 9.4 Monohybrid Cross (in corn) ; FOIL method to determine gametes for two-trait problems (p. 89) MATERIALS AND PREPARATIONS Instructions are grouped by exercise. Some materials may be used in more than one exercise. Special Requirements Living material. Tobacco seedlings for 9.1 One-Trait Crosses. Alternative corn seedlings may be used. Drosophila flies for 9.2 and 9.3 All sections ______stereomicroscope ______ hand lens ______ lens paper 9.1 One-Trait Crosses (LM pages 84-88) Color of Tobacco Seedlings (LM page 85-86) _____ tobacco seedlings (a Biokit®, is available from Carolina) The kit contains seeds, growth chambers, and germination papers for a class of thirty students. Sow seeds approximately ten days before use. The seedlings can be maintained for about a week. (The albino ones will die shortly thereafter.) Color of Corn Kernels (LM pages 86-87) _____ Corn Genetics Biokit® (Carolina Biological Supply) The kit comes with fifteen ears, marker pins, a teacher’s manual, and thirty student guides. A variety of genetic corns and student guides are available. 9.2 Two-Trait Crosses (LM pages 88-93) Color and Texture of Corn (LM pages 89-90) 49 _____ Corn Dihybrid Genetics Biokit® (Carolina 17 6380) This kit comes with fifteen ears, marker pins, a teacher’s manual, and thirty student guides. A variety of other genetic corns and student guides are available. Wing Length and Body Color in Drosophila (LM pages 91-93) _____ Results of the cross LlGg X llgg from Carolina Biological Supply (optional) _____ card, white index _____ lens paper _____ brush, camel-hair Drosophila Cross (LM page 91). If you wish students to count the flies do this: After receiving the results of the cross LlGg X llgg from Carolina Biological Supply, transfer the flies into a fresh vial that has no culture medium and freeze the flies overnight. Have students put the flies back into the vial and return to you for use by another group. As the culture bottle produces new flies, continue to freeze them for future labs. 9.3 X-Linked Crosses (LM pages 93-95) Red/White Eye Color in Drosophila _____ Results of the cross red-eyed male × heterozygous red-eyed female from Carolina Biological Supply (optional) _____ card, white index _____ lens paper _____ brush, camel-hair Drosophila X-linked cross (LM 93) If you wish students to count the flies do this: After receiving the results of the cross red-eyed male x heterozygous red-eyed female from Carolina Biological Supply, transfer the flies into a fresh vial that has no culture medium and freeze the flies overnight. Have students put the flies back into the vial and return to you for use by another group. As the culture bottle produces new flies, continue to freeze them for future labs. EXERCISE QUESTIONS 9.1 One-Trait Crosses (LM pages 84–88) Color of Tobacco Seedlings (LM pages 85-86) Experimental Procedure: Color of Tobacco Seedlings What is the expected phenotypic ratio? 3:1 = three green plants to one white plant Table 9.1 Color of Tobacco Seedlings Number of Offspring Green Color White Color Phenotypic Ratio Plate # 3:1 Plate # 3:1 Plate # 3:1 Totals 85 30 3:1 Class Data 3:1 50 Conclusions: Color of Tobacco Seedlings (LM page 86) Do your results differ from the expected ratio? yes Explain. Counting small numbers of offspring is more likely to cause a variation from the expected ratio. Was your class data closer to the expected ratio? yes Color of Corn Kernels (LM, page 86-87) Experimental Procedure: Color of Corn Kernels (LM, page 87) What is the expected phenotypic ratio? 1:1=one purple kernel to one yellow kernel Table 9.2 Color of Corn Kernels Number of Kernels Purple Color Yellow Color Phenotypic Ratio Plate # 1:1 Plate # 1:1 Plate # 1:1 Totals 60 60 1:1 Class Data 1:1 Conclusions: Color of Corn Kernels (LM page 87) Do your results differ from the expected ratio? yes Explain. Counting small numbers of offspring is more likely to cause a variation from the expected ratio. Was your class data closer to the expected ratio? yes Explain. Counting a large number of offspring is more likely to result in the expected ratio. One-Trait Genetics Problems (LM page 88) 1. In pea plants purple flowers (P) is dominant and white flowers (p) is recessive. What is the genotype of pure-breeding white plants? Pure-breeding means that they produce plants with only one phenotype. pp If pure-breeding purple plants are crossed with these white plants, what phenotype is expected? Purple plants 2. In pea plants, tall (T) is dominant and short (t) is recessive. A heterozygous tall plant is crossed with short plant. What is the expected phenotypic ratio? 1:1 3. Unexpectedly to the farmer, two tall plants have some short offspring. What is the genotype of the parent plants and the short offspring?: parents are Tt and offspring is tt 4. In horses, two trotters are mated to each other and produce only trotters and pacers are mated to each other and produce only pacers. When one of these trotters is mated to one of the pacers, all the horses are trotters. Create a key and show the cross. Key: T = trotter, t = pacer Cross: TT X tt 5. A brown dog is crossed with two different black dogs. The first cross produces only black dogs and the second cross produces equal numbers of black and brown dogs. 51 What is the genotype of brown dog? bb The first black dog? BB The second black dog? Bb 6. In pea plants green pods (G) is dominant and yellow pods (g) is recessive. When two pea plants with green pods are crossed, 25% of the offspring have yellow pods. What is the genotype of all plants involved? parents are Gg ; 50% of offspring are Gg; 25% of offspring are gg; 25% of offspring are GG. 7. A breeder wants to know if a dog is homozygous black or heterozygous black. If the dog is heterozygous, which cross is more likely to produce a brown dog Bb X bb or Bb X Bb? Explain The cross Bb X bb gives a 50% chance of a brown dog but Bb X Bb gives a 25% chance of a brown dog. 8. If the cross in #6 produces 220 plants, how many offspring have green pods and how many have yellow pods? 55 have yellow bods and 165 have green pods If the cross in #2 produces 220 plants, how many offspring are tall and how many are short? 110 are tall and 110 are short 9.2 Two-Trait Crosses (LM pages 88-93) Color and Texture of Corn (LM pages 89–90) Experimental Procedure: Color and Texture of Corn 1. Do the Punnett square in order to state the expected phenotype ratio among the offspring 9:3:3:1 (9 purple smooth to 3 purple rough to 3 yellow smooth to 1 yellow rough ) Table 9.3 Color and Texture of Corn Purple Smooth 162 78 51 291 Purple Rough 52 16 16 97 Number of Kernels Yellow Smooth 55 20 18 93 Yellow Rough 18 9 6 33 Phenotypic Ratio Sample # ____ 9:3:3:1 Sample # ____ 9:3:3:1 Sample # ____ 9:3:3:1 Totals 9:3:3:1 Class Data 9:3:3:1 These data are possible, however individual and class data will vary. Questions can be answered using these data if students do not do the experiment. Conclusions: Color and Texture of Corn (LM pages 90) Calculate the actual phenotypic ratios based on the data and record in Table 9.3. Do your results differ from the expected ratio per individual data? most likely per class data? closer to expected ratio Explain. The more offspring counted the greater the probably of achieving the expected ratio. Wing Length and Body Color in Drosophila (LM pages 91-92) What is the expected phenotypic ratio for this cross? 1:1:1:1 Table 9.4 Wing Length and Body Color in Drosophila 52 Number of Offspring Class data Phenotypes Long Long Short Short Gray ebony Gray Black 28 32 25 30 128 120 120 120 Phenotypic Ratio 1:1:1:1 1:1:1:1 Conclusions: Wing Length and Body Color in Drosophila (LM page 92) Calculate the actual phenotypic ratio based on the data and record in Table 9.4. Do the results differ from the expected ratio per individual data? yes, probably per class data not as much. Explain. The more offspring that are counted, the greater is the probability of achieving the expected ratio. Two-Trait Genetics Problems (LM pages 92-93) 1. In tomatoes, tall is dominant and short is recessive. Red fruit is dominant and yellow fruit is recessive. Choose a key for height: T= tall, t= short for color of fruit: R = red, r = yellow What is the genotype of a plant heterozygous for both traits? TtRr What are the possible gametes for this plant? TR, tR, tr, Tr 2. Using words, what are the likely parental genotypes if the results of a two-trait problem are 1:1:1:1 among the offspring? heterozygous in both traits X homozygous recessive in both traits 3. In horses, black (B) and trotting gait (T) are dominant while brown (b) and a pacing gait (t) are recessive. If a black trotter (homozygous for both traits) is mated to a brown pacer , what ratio is expected among the offspring? All black trotter 4. Two black trotters have a brown pacer offspring. What is the genotype of all horses involved? black trotter parents: BbTt brown pacer offspring: bbtt 5. The phenotypic ratio among the offspring for two corn plants producing purple and smooth kernels is 9:3:3:1. (See lab for the key) a. What is the genotype of the parental plants? PpSs What is the phenotype of the 9 offspring? Purple smooth 3 of the offsping? purple rough the other 3? yellow smooth and the 1 offspring? yellow rough 6. Which matings could produce at least some fruit flies heterozygous in both traits? Write yes or no beside each. (You do not need a key) ggLl X Ggll yes GGLl X ggLl yes GGLL x ggll yes Explain. In each cross, it is possible to choose a GgLl combination for the offspring. 7. State two new crosses that could not produce fruit flies heterozygous in both traits? GGLL X GGLL, GGLL X GgLL, GGLl X GGLl (Any combination in which the offspring must receive two capital letters for one of the traits.) ggll X ggll 53 8. Chimpanzees are not deaf if they inherit both an allele E and an allele G. A cross between two deaf chimpanzees produces only chimpanzees that can hear. What are the genotypes of of all chimpanzees involved? Parents: GGee X ggEE Offspring: GgEe 9.3 X-linked Crosses (LM pages 93-94) Red/White Eye Color in Drosophila (LM page 93-94) Complete this Punnett square and state the expected phenotypic results for this cross? females all red eyes males 1:1 Experimental Procedure: Red/White Eye Color in Drosophila (LM pages 94) Table 9.5 Red/White Eye Color in Drosophila Number of Offspring Your Data: Red Eyes White Eyes Males 16 17 Females 63 0 Class Data: Males 45 48 Females 215 0 Phenotypic Ratio 1:1 All red eyes 1:1 All red eyes Conclusions: Red/White Eye Color in Drosophila (LM page 94) Do your results differ from the expected ratio per individual data? yes per class data? not as much Explain. The more offspring that are counted, the greater is the probability of achieving the expected ratio. In the space provided, do a Punnett square to calculate the expected phenotypic results for the cross XRY × XrXr. females all red eyed; males all white-eyed X-linked Genetics Problems (LM page 95) 1. State the genotypes and gametes for each of these fruit flies: genotype white-eyed male Xr Y white-eyed female XrXr red-eyed male XRY homozygous red-eyed female XRXR R r heterozygous red-eyed female X X gamete(s) Xr , Y Xr XR, Y XR R r X ,X 2. What are the results if a white-eyed female is crossed with a red-eyed male? Males All white-eyed males Females All red eyes 3. Regardless of any type cross, do white-eyed males inherit the allele for white eyes from their father or mother? mother Explain Males receive a Y from their father 4. In sheep, horns are sex linked and H = horns and h = no horns. Using symbols, what cross do you recommend if a farmer wants to produce hornless males? XHY X XhXh 54 5. In Drosophilia, bar eye (B) is dominant and no bar eye (b) is recessive. What are the results of these crosses? Bar eyed male X non-barred eyed female XBY X XrXr = XrY ;All no-barred males; 1:1 Bareyed: nonbarred eyed females Bar eyed male X heterozygous female XBY X BXb= Males 1:1 and Females all bar-eyed; non-bar eye male X heterozygous female 6. A female fruit fly has white eyes. What is the genotype of the father? XrY What could be the genotype of the mother? XrXr or XRXr 7. In a cross between fruit flies, all the males have white eyes and the females are 1:1. What is the genotype of the parents? female parent XrXr male parent XRY 8. In a cross between fruit flies, a white-eyed male and red-eyed female produce no offspring that have white eyes. What is the genotype of the parents. Male parent XrY Female parent XRXR LABORATORY REVIEW 9 (LM page 96) 1. If offspring exhibit a 3:1 phenotypic ratio, what are the genotypes of the parents? Aa x Aa 2. In fruit flies, which of the characteristics you studied was X-linked? red/white eye color 3. If offspring exhibit a 9:3:3:1 phenotypic ratio, what are the genotypes of the parental generation? AaBb x AaBb 4. If a cross results in 90 long-winged flies to 30 short-winged flies, what are the phenotypes of the parents? long-winged 5. Briefly describe the life cycle of Drosophila. The adults reproduce by laying eggs. The eggs hatch into larvae that feed. The larvae form pupae, in which the tissues are reorganized into an adult. 6. In the cross AaBb X aaBB, what are the gametes for AaBb? AB, Ab, aB, ab For aaBb? aB, ab What are the genotypic results for this cross? AaBB, AaBb (2), Aabb, aaBB, aaBb (2), aabb 7. What is the genotype of a white-eyed male fruit fly? XrY 8. Suppose you counted 40 green tobacco seedlings and 2 white tobacco seedlings in one agar plate. Do your results show that both parent plants were heterozygous for the color allele? Yes, because only Aa X Aa can produce an offspring that is aa. 9. Suppose you counted tobacco seedlings in six agar plates, and your data were as 55 follows: 125 green plants and 39 white plants. What is the phenotypic ratio? 3:1 10. Suppose that students in the laboratory periods before you removed some of the purple and yellow corn kernels on the ears of corn as they were performing the Experimental Procedure. What effect would this have on your results? An accurate 9:3:3:1 ratio could not be obtained due to incomplete data. 56 Laboratory 10 DNA Biology and Technology (LM pages 97-110) Fourth Edition A new procedure is used for isolating DNA in a test tube. MATERIALS AND PREPARATIONS Instructions are grouped by exercise. Some materials may be used in more than one exercise. 10.1 DNA Structure and Replication (LM pages 98-100) _____ model, DNA; model kit, DNA-RNA; or puzzle kit, DNA 10.2 RNA Structure (LM pages 101-02) _____ puzzle kit, DNA 10.3 DNA and Protein Synthesis (LM 102 -105) _____ model kit, DNA-RNA Protein Synthesis Kits and models. For Sections 10.1 to 10.3, DNA kits are available from Carolina Biological Supply and Lab Aids, from which students construct models. The kits vary in degree of sophistication and in price. Descriptions and price information for the Carolina products can be found in the “Genetics” section of the Carolina catalog. Alternatively, students can simply use the figures in the lab manual to gain an understanding of the concepts. 10.4 Isolation of DNA and Biotechnology (LM page 106-107) DNA isolation _____ safety goggles (See Carolina’s Safety: Face Protection Section) _____ latex gloves and/or nonlatex gloves (See Carolina’s Safety: Hand Protection Section) _____ lab coats (See Carolina’s Safety: Body Protection Section) or other clothing protect _____ _____ _____ _____ _____ _____ _____ _____ slice of fruit or vegetable mortar and pestle large, clean test tube on ice 0.9% NaCl dishwasher detergent (e. g., Blue Dawn) transfer pipets ice-cold ethanol small, clean test tube 57 _____ For laboratory: _____ _____ _____ _____ _____ _____ phenol red test tubes, large test tube rack ice-water bath transfer pipets 0.9% NaCl solution 95% ethanol, ice cold (5 ml per student group) Gel Electrophoresis (LM page 106-107) Note: If desired, students can gain an understanding of the gel electrophoresis process by using the description and figures in the Lab Manual, rather than performing the actual procedure. _____ horizontal gel electrophoresis apparatus: _____ power supply _____ cables _____ electrophoresis chamber with gel _____ Electrophoresis DNA Separation Kit Horizontal gel electrophoresis apparatus.Biological suppliers have various types of electrophoresis apparatuses for sale. Biostar Corporation (P.O. Box 5756, Lafayette, In 47903) has Quadracell units (QEC-100) and power supply (MAB-125), which allow four gels of four lanes each per unit. Electrophoresis DNA Separation Kit If a kit is not obtained, the following supplies will be needed: Electrophoresis buffer (optional). If you have purchased a kit, the electrophoresis buffer will be included. Otherwise, make up a sterile 5% stock TBE buffer as follows: 54 g of Tris base (Tris aminomethane buffer), 27.5 g of boric acid, 20 ml of 0.5 M EDTA (disodium ethylene diamine tetraacetate 2H2O) (pH 8.0). Note: The wells also can be loaded before adding the buffer. Then they will need to be sealed with agarose solution. Agarose solution. Agarose powder can be purchased from biological suppliers. It also comes as part of a molecular biology experiment package, along with instructions for making the gel slab. Gel slabs. Gel slabs can be used immediately, or they can be covered with plastic and left overnight (or longer) in the refrigerator. Micropipettes and micropipette tips. Either adjustable or fixed pipettes are recommended. When using adjustable pipettes, you need only one (5–50 ml) per setup, with one kind of tip. To pipette 100 ml, just use the 50 ml adjustment level twice. (VWR Scientific, with offices in many major cities, is a good supplier of adjustable pipettes.) 58 The tip can be cleaned by rinsing three times, but when working with bacteria, using a new/sterile tip each time is preferable. (Tips can be reused after rinsing and resterilization in their dispenser boxes.) 10.5 Detecting Genetic Disorders ____ paper and pencil EXERCISE QUESTIONS 10.1 DNA Structure and Replication (LM pages 98–100) Observation: DNA Structure (LM page 99) 1. Label phosphate, base pair, and deoxyribose in your drawing. See Figure 11.2B in text Table 10.1 Base Colors In Figure 10.1 Cytosine red Thymine purple-blue Adenine gold Guanine green In Your Kit 3. What type of molecules make up the backbone (uprights of ladder) of DNA (Fig. 10.1)? sugar and phosphate molecules 4. Label a hydrogen bond in Figure 10.1. The label goes on the only write-on line available. Dashes are used to represent hydrogen bonds in Figure 10.1 because hydrogen bonds are weak. 5. Notice … that the base A is always paired with the base T, and the base C is always paired with the base G. 6. In Figure 10.1, what molecules make up the rungs of the ladder? hydrogen-bonded bases adenine pairs with thymine; cytosine pairs with guanine 7. Why is DNA also called a double helix (Fig. 10.1)? The two strands making up DNA’s ladder configuration twist around one another in the form of a helix. DNA Replication (LM pages 99-100) Observation: DNA Replication 1. What bonds are broken in order to unzip the DNA strands? hydrogen bonds 4. Are your molecules identical? yes 5. Because of complementary base pairing, each new double helix is composed of an old strand and a new strand. Write old or new in 1–10, Figure 10.2a, b, and c. 1. old; 2. old; 3. old; 4. new; 5. new; 6. old; 7. old; 8, new; 4. new; 10. old Why is DNA replication called semiconservative? Because each new double helix is composed of an old (parental) strand and a new (daughter) strand. 6. Does replication provide a means for passing DNA from cell to cell and organism to organism? yes Explain. By replicating (making a copy of itself) daughter cells receive a copy of the DNA. 59 Table 10.2 DNA Replication Old strand G G G T T C C A T T A A A T T C C A G A A A T C A T A New strand C C C A A G G T A A T T T A A G G T C T T T A G T A T 10.2 RNA Structure (LM Pages 101-102) 1. Describe the backbone of an RNA molecule. RNA, like DNA, has a sugar phosphate backbone. 2. Where are the bases located in an RNA molecule? to the side Observation: RNA Structure (LM pages 101-102) 1. Label the ribose (the sugar in RNA), the phosphate, and the base in your drawing and in 1–3, Figure 10.3. 1. phosphate 2. base 3. ribose; Table 10.3 Base Colors In Figure 10.3 Cytosine red Uracil purple-blue Adenine gold Guanine green In Your Kit Table 10.4 DNA Structure Compared with RNA Structure DNA RNA Sugar Deoxyribose Ribose Bases Adenine, guanine, thymine, cytosine Adenine, guanine, uracil, cytosine Strands Double stranded with base pairing Single stranded Helix Yes No Complementary Base Pairing (LM, p. 102) Complete Table 10.5 to show the complimentary DNA bases for the RNA bases Table 10.5 DNA and RNA Bases RNA Bases C U DNA Bases G A A T G C 10.3 DNA and Protein Synthesis (LM pages 103-105) What is the role of each of these participants in protein synthesis. DNA: stores information (i.e., proper sequence of amino acids) mRNA: carries information to ribosomes tRNA: brings amino acids to ribosomes Transcription (LM page 103) 60 Label Figure 10.4. 1. RNA polymerase; 2. mRNA transcript Observation: Transcription Table 10.6 Transcription DNA T A C A C G A G C AA C T A A C A T mRNA A U GU G C U C G U U G AU U G U A 4. Locate the end of the strand that will move to the ribsosomes in the cytoplasm. 5’end Translation (LM pages 104–105) Label Figure 10.5. 1. amino acid; 2. tRNA; 3. anticodon Observation: Translation (LM pages 104) 1. Using the mRNA sequence given in Table 10.7, number the tRNA–amino acid complexes in the order they will come to the ribosome. Figure 10.6 : Top row : 1, 2, 5; Bottom row : 4, 3, 6, 7 2. Complete Table 10.7. Why are the codons and anticodons in groups of three? The genetic code is a triplet code. Table 10.7 Translation mRNA codons AUG CCC GAG* GUU GAU UUG UCU tRNA anticodons UAC GGG CUC CAA CUA AAC AGA Amino acid Met Pro Glu Val Asp Leu Ser *Both GAG and GAA code for Glu 10.4 Isolation of DNA and Biotechnology (LM page 106) Experimental Procedure: Isolation of DNA Answer the following questions(page 107) 1. What is biotechnology? Biotechnology is the manipulation of DNA for the benefit of human beings and other organisms. 2. Speculate how the ability to isolate DNA and run gel electrophoresis of DNA related to biotechnology. Isolating DNA and performing gel electrophoresis shows that DNA is subject to laboratory procedures the same as any other molecule. 3. Name a biotechnology product someone you know is now using or taking as a medicine. Answers will vary. 10.5 Detecting Genetic Disorders (LM pages 107–109) Genetic Sequence for Sickle Cell Disease 1. In what three-DNA-base sequence does HbA differ from HbS? HbA CTC HbS CAC 2. What are the codons for these three bases? HbA GAG HbS GUG 3. What is the amino acid difference? HbA glu HbS val Detection of Sickle-Cell Disease by Gel Electrophoresis (LM pages 109) 61 2. In Figure 10.10 which lane contains only HbS, signifying that the individual is HbSHbS. Lane 2 3. Which lane contains only HbA, signifying that the individual is HbAHbA. Lane 1 4. Which lane that contains both HbS and HbA, signifying that the individual is HbAHbS. Lane 3 Detection by Genomic Sequencing What are the chances that this couple will have a child with sickle-cell disease? Both partners are heterozygous; therefore the chance this couple will have a child with sickle-cell disease is 25%. Summary: Detecting Genetic Disorders (LM page 109) • What two methods of detecting genetic disorders were described in this section? Gel electrophoresis; genomic sequencing • Which method is more direct and probably requires more expensive equipment to do? Genomic sequencing • Which method probably preceded the other method as a means to detect sicklecell disease? Gel electrophoresis LABORATORY REVIEW 10 (LM page 110) 1. Explain why DNA is said to have a structure that resembles a ladder. The paired bases are the rungs of the ladder and the sugar-phosphate backbones are the supports. 2. Do the two DNA double helices following DNA replication have the same, or a different, composition? Same 3. How is complementary base pairing different when pairing DNA to DNA than when pairing DNA to mRNA? Uracil replaces thymine in RNA. 4. Explain why the genetic code is called a triplet code. Every three bases stands for one of the twenty amino acids in DNA. 5. What role does each of the following molecules play in protein synthesis? a. DNA Contains inherited genetic information. b. mRNA Contains codons c. tRNA Has a specific anticodon d. Amino acids The unit molecules of a protein 6. Which of the molecules listed in question 5 are involved in transcription? DNA and mRNA 7. Which of the molecules listed in question 5 are involved in translation? mRNA, tRNA, and amino acids. 8. What is the purpose of gel electrophoresis? The separate DNA molecules or amino acids. 62 9. Why does sickle-cell hemoglobin (HbS) migrate slower than normal hemoglobin (HbA) during gel electrophoresis? HbS contains valine (no charge) instead of glutamate (has charge). 10. Below is a sequence of bases associated with the template DNA strand: TAC CCC GAG CTT a. Identify the sequence of bases in the mRNA resulting from the transcription of theabove DNA sequence. AUG GGG CUC GAA b. Identify the sequence of bases in the tRNA anticodon that will bind with the first codon on the mRNA identified above. UAC 63 Laboratory 11 Genetic Counseling (LM pages 111–124) Fourth Edition This lab was rewritten to begin with genetic inheritance and provide students with many genetic problems pertaining to human disorders. Multiple allele inheritance and the use of blood type to determine paternity has been added to increase interest. MATERIALS AND PREPARATIONS 11.2 Patters of Genetic Inheritance (LM pages 114-119) Multiple Alleles (page 118) _____ Kit to determine paternity available from Carolina Biological Supply EXERCISE QUESTIONS 11.1 Determining the Genotype (LM pages 112-113) Autosomal Dominant and Recessive Traits (LM page 112-113) 1. What is the homozygous dominant genotype for type of hairline? WW What is the phenotype? widow's peak 2. What is the homozygous recessive genotype for finger length? ss What is the phenotype? long fingers 3. Why does the heterozygous individual Ff have freckles? Freckles is dominant and they have one dominant allele. 4. Maria and the members of her immediate family have attached earlobes. Her maternal grandfather has unattached earlobes. What is the genotype of her maternal grandfather? Ee Explain. Maria's mother has the genotype ee (results in the recessive phenotype), therefore her maternal grandfather, who has unattached earlobes, must be Ee. 5. Moses does not have a bent little finger, but his parents do. Deduce the genotype of his parents. Moses genotype is ll; therefore, his parents who have bent little fingers must be Ll. 6. Manny is adopted. He has hair on the back of his hand. Could both of his birth parents have had no hair on the back of the hand? No Explain. The presence of hair on the back of the hand is a dominant characteristic; at least one parent had to have hair on the back of the hand for Manny to have it. 7. Simona and her husband have widow peaks One child has a widow’s peak and the other does not. Give the possible phenotype of all persons involved. Isabella and her husband Ww X Ww Child with straight hairline ww Child with widow’s peak WW or Ww 64 Experimental Procedure: Human Traits (page 113) 4. Are dominant phenotypes always the most common in a population? No Explain. Phenotypes depend on inherited alleles and not on whether traits are dominant or recessive. Table 11.1 Autosomal Human Traits Answers may vary according to the class members. Students may not know whether they are homozygous dominant or heterozygous. If so, they can use A? for their genotype, for example. 11.2 Patterns of Genetic Inheritance (LM pages 114-119) Inheritance of Genetic Disorders In Figure 11.3a, ¼ offspring have the phenotype = 25% chance ¾ offspring have the phenotype = 75% chance In Figure 11.3b, ½ offspring have the phenotype = 50% chance 1a. With reference to Figure 11.3a, if a genetic disorder is recessive and both parents are heterozygous (Aa), what are the chances that an offspring will have the disorder? 1 in 4 (25%) b. With reference to Figure 11.3a, if a genetic disorder is dominant and the parents are heterozygous (Aa), what are the chances that an offspring will have the disorder? 3 out of 4 (75%) 2a. With reference to Figure 11.3b, if parents are heterozygous (Aa) by homozygous recessive (aa), and the genetic disorder is recessive, what are the chances that an offspring will have the disorder? 50/50 (50%) b. With reference to Figure 11.3b, if the parents are heterozygous (Aa) by homozygous recessive (aa), and the genetic disorder is dominant, what are the chances that an offspring will have the disorder? 50/50 (50%) Autosomal Disorders (LM pages 115-116) 1. Neurofibromatosis is a dominant disorder. If a heterozygous (Aa) woman reproduces with a homozygous (aa) normal man, what are the chances a child will have neurofibromatosis? 50/50 (50% ) 2. Cystic fibrosis is a recessive disorder. A carrier is an individual that appears to be normal but carries a recessive allele for a genetic disorder. A man and a woman are both carriers (Aa) for cystic fibrosis. What are the chances a child will have cystic fibrosis? 1 in 4 (25%) 3. Huntington disease is a dominant disorder. Drina is 25 years old and as yet has no signs of Huntington disease. Her mother does have Huntington disease (Aa), but her father is free (aa) of the disorder. What are the chances that Drina will develop Huntington disease? 50/50 (50%) 4. Phenylketonuria (PKU) is a recessive disease. Mr. and Mrs. Martinez appear to be normal, but they have a child with PKU. What are the genotypes of Mr. and Mrs. Martinez? Both are heterozygous (Aa) for the disease. 5. Tay-Sachs is an autosomal recessive disorder. Is it possible for two individuals who 65 do not have Tay-Sachs to have a child with the disorder? Yes. Explain. If both parents are heterozygous carriers (Aa) for the disease each child has a 25% chance of Tay-Sachs. X-Linked Disorders (LM page 116-117) Does a color-blind male give his son a recessive-bearing X or a Y that is blank for the recessive allele? Y 1a. What is the genotype for a color-blind female? XbXb How many recessive alleles does a female inherit to be color blind? two b. What is the genotype for a color-blind male? XbY How many recessive alleles does a male inherit to be color blind? One 2a. With reference to Figure 11.4a, if the mother is a carrier (XBXb) and the father has normal vision (XBY), what are the chances that a daughter will be color blind? None b. A daughter will be a carrier? 50/50 (50%) A son will be color blind? 50/50 (50%) 3a. With reference to Figure 11.4b, if the mother has normal vision (XBXB) and the father is color blind (XbY) , what are the chances that a daughter will be color blind? none b. A daughter will be a carrier? 100% A son will be color blind? none X-Linked Genetics Problems (LM page 117) 1.A woman with normal color vision (XBXb) whose father was color blind (XbY), marries a man with normal color vision (XBY) What genotypes could occur among their offspring?. Their children could be XBXB, XBXb, XBY, or XbY. What genotypes could occur if it was the normal-visioned man’s father who was color blind? This means his wife is not a carrier and since both parents are normal, the children could be only XBX or XBY. 2. Antonio’s father is color blind (XbY) but his mother is not color blind (XBXb or XBXB). Is Antonio necessarily color blind? no How so? Even if his mother is XBXb he could inherit the XB. Could he be color blind? Yes How so? If his mother is XBXb he could inherit the Xb. 3. Make up a cross involving hemophilia that could be answered by a Punnett square, as in Figure 11.4a or b. For example, A normal man reproduces with a carrier female. What are the chances that a son will have hemophilia. What is the answer to your genetics problem? For this cross, the answer is 50%. Multiple Alleles (LM page 118-119) Experimental Procedure: Using Blood Type to Help Determine Paternity If a person is AB+, which wells would show agglutination? All three wells. Table 11.2 Blood Types of Involved Persons Mother* Child* Wanda Sophia #1 Blood type BAB+ A*Your instructor may have you confirm these results. Father ? #2 B- #3 AB+ Conclusion (page 119) 1. Noting that only father #3 could have given Sophia the Rh antigen, from whom did she receive the IB allele? mother From which parent did she receive the IA allele? father #3. Is there any other possible interpretation to the results of blood typing? No. 66 Blood Typing Problems (page 119) 1. A man with type A blood reproduces with a woman who has type B blood. Their child has blood type O. Using IA, IB, and i give the genotype of all persons involved: man IA i woman IBi, and child ii. 2. If a child has type AB blood and the father has type B blood, what could the genotype of the mother be? IAIA or IAi 3. If both mother and father have type AB blood, they cannot be the parents of a child who has what blood type? Type O blood 4. What blood types are possible among the children if the parents are IAi X IBi . (Hint: do a Punnett Square using the possible gametes for each parent.) Types A, B, AB and O. 11.3 Genetic Counseling (LM page 119-123) Observation: Sex Chromosome Anomalies (LM pages 120) Label each karyotype in Figure 11.5. a. Poly X; 3 Xs are present b. Turner; one X is present c. Klinefelter; X and Y are present d. Jacob; XYY are present Determining the Pedigree (LM pages 121-–122) Pedigree Analyses 1. a. Notice that neither of the original parents is affected but several children are affected. This could only happen if the trait were autosomal recessive.. b. What is the genotype of the following individuals? Generation I, individual 1: Aa This individual has to be heterozygous because some of the children are affected. Generation II, individual 1: aa This individual has to be homozygous recessive because he is affected. Generation III, individual 8: Aa This has to be the case because the mother is homozygous recessive, and the individual has to inherit at least one of her recessive alleles. 2. a. Notice that only males are affected. This could only happen if the trait were Xlinked recessive. b. What is the genotype of the following individuals? Generation I, individual 1: XAXa This female has to be a carrier because she has an affected son. Generation II, individual 8: XAX? Unable to determine whether this female is a carrier or not because she had no children. Generation III, individual 1: XAY This male is unaffected; therefore, he must have received a dominant allele. Construction of a Pedigree (LM page 123) 2. Choose a key for this trait? a = normal eyelashes; A = double row of eyelashes 4. Which pattern is correct? autosomal dominant 5. Use correct genotypes to show a cross between Henry and Isabella and calculate the expected phenotypic ratio among the offspring: Aa × aa; 1:1 6. What are the percentage chances of Henry and Isabella having a child with double eyelashes? 50% Explain why each child has the same chance for double eyelashes. 67 Because each child has a 50% chance of receiving either and A or a from their father (Henry). LABORATORY REVIEW 11 (LM page 124) 1. If an individual exhibits the dominant trait, do you know the genotype? Why or why not? The individual has a dominant allele but may also have a recessive allele for the trait. 2. Isabella’s father does not have freckles, but Mary does. What genotypes could Mary’s mother have? FF of Ff 3. What are the chances two individuals with an autosomal recessive trait will have a child with this trait? 100% 4. Show a cross that would produce a phenotypic ratio of 1:1 among the offspring. Aa X aa 5. If the parents are heterozygous for cystic fibrosis, what are the chances of a child having cystic fibrosis? 3:1 6. Tom has blood type AB. Show all possible genotypes for this type blood. IAIB 7. Mary has blood type A and Don has blood type B; can they be the parents of a child with type O blood? Show why or why not. Yes because Mary could be IAi and Don could be IBi 8. What syndrome is inherited when an egg carrying two X chromosomes is fertilized by a sperm carrying one Y chromosome? XXY Klinefelter syndrome 9. What is the inheritance pattern in a pedigree if the parents are not affected and a child is affected? Give a genotype for all persons. Autosomal recessive. Parents are Aa and child is aa 10. If only males are affected in a pedigree, what is the likely inheritance pattern for the trait? X-linked recessive. Draw a three-generation pedigree showing the inheritance of the trait from an affected grandfather to an affected grandson. (No spouses are affected). Grandfather: XaY Daughter XAXa Grandson XaY 68