Salinity and Density Lab - Aimee Clark`s E
advertisement

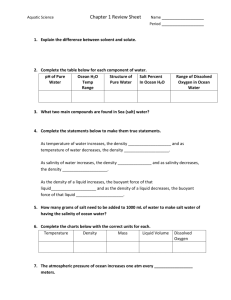
SALINITY AND DENSITY and DEEP OCEAN CURRENTS LAB Science Inquiry Ms. Clark October 31, 2012 GOALS / OBJECTIVES Ocean currents arise in many different ways. For example, wind pushes the water along the surface to form wind-driven currents. Deep ocean currents are caused by differences in water temperature and salinity. In this experiment, the students will hypothesize the cause of ocean currents and then develop a model to explain the role of salinity and density in deep ocean currents. STANDARD SCI.6.1 2010 - Physical Science Explain that all objects and substances in the natural world are composed of matter in different states with different properties. (6.1.1, 6.1.2, 6.1.3) Understand that there are different forms of energy with unique characteristics. (6.1.4, 6.1.5, 6.1.6, 6.1.7) MATERIALS / SUPPLIES Glass beakers (2 large and 2 small per group) Salt Food coloring Measuring spoons Water (hot and cold) Stir sticks ESTIMATED TIME NEEDED: One class period SAFETY STATEMENT: This is a safe experiment with very little risk of injury. SALINITY AND DENSITY and DEEP OCEAN CURRENTS LAB Science Inquiry Ms. Clark October 31, 2012 ACTIVITY/PROCESS Introduction Display the maps of (1) wind-driven ocean currents, (2) sea surface temperature, and (3) surface salinities of the oceans. Have the students look for relationships between sea surface temperature, salinity, and the locations of warm and cold currents. Ask the students to write a hypothesis that explains these relationships, if possible. Conduct the following experiment to learn more about the relationship between salinity and deep ocean currents. Experimental Procedure 1. Fill large beaker with water to within 2 inches from the top 2. Dissolve a Tbs. of salt in ¼ cup water in the small beaker and add a drop of blue food coloring. 3. Slowly pour the “salt” water into the side of the larger beaker. 4. Use the second set of beakers to repeat the experiment. This time, the large beaker will have cold water, and the small, “blue” will have hot water. 5. Record observations in Science notebook. 6. You may extend the activity mixing different salinity levels and different water temperatures, or add layers using different food colors. REFLECTION / FOLLOW ACTIVITY What Happened: CONCEPTS Salt water is more dense than fresh water, and is therefore heavier. When ocean water evaporates, the water becomes more dense because most of the salt remains in the water. In some regions of the ocean, circulation is based upon the mixing between more dense surface water and less dense layers of deeper water. Explanation Thermohaline circulation is the name for currents that occur when colder, saltier water sinks and displaces water that is warmer and less dense. In this activity, you examined the relationship between salinity and deep ocean currents. In Earth's equatorial regions, surface ocean water becomes saltier as the water, but not the salt, evaporates. However, the water is still warm enough to keep it from sinking. Water that flows towards the poles begins to cool. In a few regions, especially in the North Atlantic, cold salty water can sink to the sea floor. It travels in the deep ocean back towards the equatorial regions and rises to replace water which is moving away at the surface. This whole cycle is very important in regulating climate as it transports heat from the equatorial regions to polar regions of Earth. The full cycle can take a thousand years to SALINITY AND DENSITY and DEEP OCEAN CURRENTS LAB Science Inquiry Ms. Clark October 31, 2012 complete. Follow up activity: Vocabulary for Science Notebook density: mass per unit volume of a substance. Usually expressed as grams per cubic centimeter. hypothesis: an assumption made to account for or relate known facts. model: system of data, inferences, and relationships, presented as a description of a process or entity. salinity: a measure of the quantity of dissolved solids in ocean water. Formally, it is the total amount of dissolved solids in ocean water in parts per thousand by weight after all carbonate has been converted to oxide, the bromide and iodide to chloride, and all the organic matter oxidized. temperature: a direct measure of the average kinetic energy of the molecules of a substance. The degree of hotness or coldness of anything. current: a smooth and steady onward movement of a fluid (i.e., liquid or gas). The part of any body of fluid that has a continuous onward movement. thermohaline circulation: the vertical movement of ocean water driven by density differences resulting from the combined effects of variations in temperature and salinity. Research Questions for Science Notebook Is salt water heavier or lighter (higher or lower in density) than fresh water? Make sure that you explain your answer in terms of the results that you obtained from your experiment. If evaporation causes surface water to be salty, where would you expect ocean water to be very dense? Does the density of ocean water have any relationship to the temperature of ocean water? Differentiation: Have students compare the map of sea surface temperature to the map of surface salinity. Based on what they’ve learned from the animation and this activity, what combination of temperature and salinity favors the sinking of ocean water? Think about the parts of the ocean where cold salty ocean water tends to sink. Can fresh water from nearby land masses affect the salinity there? How might the influx of fresh water affect the process? What about global warming and the associated melting of polar ice?