AP Chemistry- 9 Week Summary Chapter One: Introduction: Matter

AP Chemistry- 9 Week Summary
Chapter One: Introduction: Matter and Measurement
Definition of chemistry
Chemistry applications
Definitions of matter,
Characteristics of mixtures
Definition of physical & chemical change
Scientific Notation*
Significant figures*
Math functions using significant figures
Density calculations*
Unit Conversions using dimensional analysis*
Chapter Two: Atoms, Molecules and Ions
Define atom
Properties of protons, neutrons, electrons
Atomic Mass
Isotopes
Define groups/families on the periodic table
Define chemical formula, formula unit
Write balanced formula unit (ionic)
Calculate empirical and molecular formulas*
Chapter Three: Chemical Reactions
Write/balance equations
Solution process & solubility
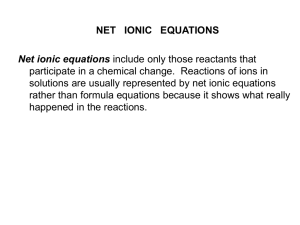
Neutralization reaction and net ionic
Redox Reaction and net ionic equations
Predict products based on the type of reaction
Divisions of chemistry
Scientific method
States of matter,
Classification of matter
Atomic structure
Atomic number
Calculate average atomic mass*
Organization of the Periodic table
Family properties
Recognizes ions and charges
Write molecular formulas (covalent)
Identify common acids
Qualitative vs. quantitative
Accuracy and precision
Metric prefixes*
SI units of measure*
Qualitative vs. Quantitative
Conversion Kelvin/Celsius*
Identify strong electrolytes
Write net ionic equations
Oxidation Numbers*
Apply solubility rules & activity series
½ reactions for redox
Chapter Four: Stoichiometry
Chemical Analysis
Apply mass relationship to chemical equations*
Theoretical and % yield
Preparation of Solutions & dilutions
Aqueous Stoichiometry
Empirical formula based on combustion
Limiting reactants
Solution Concentration (Molarity)
Beer’s Lambert Law
Titration
*Be able to solve problems by performing calculations (similar to previous tests and worksheets).