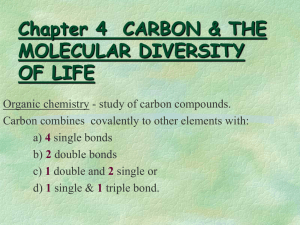Yeemoo Chai 1/17/14 Organic chemistry Organic: if a substance has
advertisement
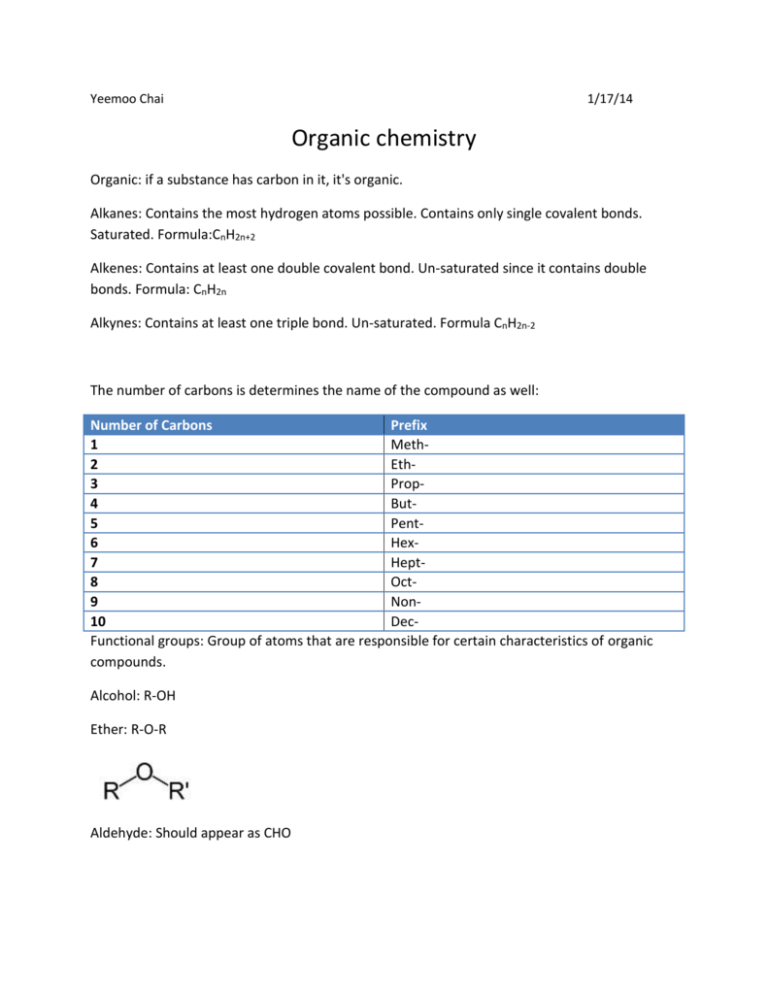
Yeemoo Chai 1/17/14 Organic chemistry Organic: if a substance has carbon in it, it's organic. Alkanes: Contains the most hydrogen atoms possible. Contains only single covalent bonds. Saturated. Formula:CnH2n+2 Alkenes: Contains at least one double covalent bond. Un-saturated since it contains double bonds. Formula: CnH2n Alkynes: Contains at least one triple bond. Un-saturated. Formula CnH2n-2 The number of carbons is determines the name of the compound as well: Number of Carbons Prefix 1 Meth2 Eth3 Prop4 But5 Pent6 Hex7 Hept8 Oct9 Non10 DecFunctional groups: Group of atoms that are responsible for certain characteristics of organic compounds. Alcohol: R-OH Ether: R-O-R Aldehyde: Should appear as CHO Ketone: Appears as R'-CO-R Carboxylic acid: Should appear as COOH Ester: C-OOR'. Not to be confused with Esther Amine: R3N. Other than Amino or Nitro, which we'll be seen later, any functional group with Nitrogen is an Amine. Akyl groups: These functional groups branch off of the main chain of carbons Methyl: -Ch3 Ethyl: CH2-CH3 n-propyl: -CH2-CH2-CH3 n-Butyl: -CH2-CH2-CH2-CH3 Isopropyl: t-Butyl: Names of Common Substituent groups Amino: NH2 Fluoro: -F Chloro: -Cl Bromo: -Br Iodo: -I Nitro: -NO2 Vinyl: -CH=CH2 Naming Organic compounds: The prefix should equal the number of carbons, and the ending should be based on the kinds of bonds in the compound (alkane, alkyne, alkene). Provide the carbon number for subsistent and double/triple bonds. Use commas between numbers and dashes between words and numbers. DO NOT LEAVE SPACES IN THE NAME. If you leave spaces in the name, Albert Einstein will find pictures of your dead grandma and burn it to ashes. Determine the names of these compounds based on their formulas. 1. C1H2 2. C4H6 3.C5H12 4. C8H18 5. C10H18 6. C7H14 Name the following compounds 7. 8. Determine what functional group is in the following compounds. 9. 10. quick answer sheet. 1. Methene 2. Butyne 3. Pentane 4. Octane 5.Decyne 6. Heptene 7. 2,3-Dimethylpentane 8. 2-methyl-2-pentane 9. Alcohol 10. Carboxylic acid