macroscopic manifestation of local disorder in mixed crystals and
advertisement

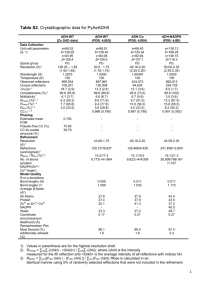
MACROSCOPIC MANIFESTATION OF LOCAL DISORDER IN MIXED CRYSTALS AND ALLOYS Yu.Rosenberg(1), V.Sh.Machavariani(2), A.Voronel(2), S.Garber(2), A.Rubstein(2), A.I.Frenkel(3), E.A.Stern(4) (1) Wolfson Center for Materials Research, Tel-Aviv University, 69978, Israel. (2) Raymond and Beverly Sackler School of Physics and Astronomy Tel-Aviv University, 69978, Tel-Aviv, Israel. (3) Materials Research Laboratory, University of Illinois at Urbana - Champaign. (4) Physics Department, Box 351560, University of Washington, Seattle, WA 98195-1560, USA. Abstract: A correlation between precise X-ray diffraction patterns and atomic size mismatch in disordered mixed crystals (alloys and ionic crystals) is observed. The anisotropy of the elastic moduli has been taken into account for evaluation of the strain energy density of the mixed crystals revealed in XRD measurements. The novel improved procedure of microstrain estimation relevant for metallic alloys is developed. The precursor of the phase transformation for a quenched disordered Au-Cu alloy is identified. PACS Numbers: 61.10. i; 61.72.Dd; 61.72.Hh; 78.30.Ly INTRODUCTION It is widely accepted that in the first approximation the lattice parameter of a mixed crystal or alloy varies linearly with the composition (Vegard's law). The real structures deviate from this oversimplified view considerably. In our extended study of mixed ionic salts with atomic size mismatch RbBr-KBr [1,2], RbCl-RbBr [1,2,3], and AgCl-AgBr [4] strong deviations of the local structure obtained by X-ray absorption fine structure (XAFS) technique from the average structure obtained by X-ray diffract ion (XRD) measurements have been revealed. The equilibrium atomic positions have been found by the XAFS analysis to be shifted from the periodic lattice sites, ascertained by diffraction. Our further XAFS investigation of the disordered metallic alloy AuxCu1-x (Ref. [5]) also reveals considerable deviations of the interatomic distances from those predicted by XRD. In our present careful XRD investigation we are trying to find the characteristic features of diffraction patterns of crystals with disorder ascertained by XAFS. The linewidths as a function of diffraction angle have been carefully measured for different concentrations of Au-Cu, Au-Ag, RbBr-RbCl and AgBr-AgCl. It has been found that a clear correlation exists between the built in atomic size disorder and the XRD line broadening in all the investigated mixed crystals. EXPERIMENTAL The Au-Cu alloys were quenched to avoid phase separation. The details of samples' preparation were presented in Ref. [1,2,3,4,5]. Homogeneity of the alloys and mixtures was verified by XRD and no trace of phase separation or superstructure were observed for all the concentrations. The compositions of the mixed samples were established by energy 311 dispersive spectoscopy with a scanning electron microscope. All specimens were found to be homogenious within the accuracy of 1%. XRD data were collected in the range 30-120 2 range with Cu K-radiation on : powder diffractometer "Scintag" equipped with a liquid nitrogen cooled Ge solid state detector. Peak positions and widths of Bragg reflections were determined by self-consistent profile fitting technique with Pearson VII function [6]. Contributions of K2 radiation were subtracted from the total profiles; the obtained results correspond to only the K1 component of the K-doublet. Lattice constant computation was carried out by a reciprocal lattice parameters refinement. The determined lattice constants are presented in Figures 1a and 2a as deviations from the Vegard's law. The Williamson-Hall [7] approach allows us to separate two different causes for a line broadening. It is assumed usually [8] that the broadening of Bragg reflection (hkl) originated from a finite grain size of the polycrystal follows the Sherrer equation: = /( cos hkl) (1) where is the X-ray wavelength, hkl is a Bragg angle, is a mean "effective" size of coherent scattering region normal to the reflecting planes. If this is the only reason for the broadening then the value ( cos hkl) should be independent of the diffraction angle. However, the real situation is different because of a persistence of the additional stress-induced broadening which is given by the Wilson formula: = 4 tan hkl (2) Here is a dimensionless value ("microstrain") which is usually assumed to be proportional to a square root of density of dislocations. Assuming that the contributions to the Bragg peak are mutually independent and both have a Cauchy-like profile, one gets for the total reflection width hkl: hkl = + = /( cos hkl) + 4 tan hkl (3) Plotting now the measured value (hkl cos hk) as a function of the argument (4 sin hkl) one can estimate microstrain from the slope of the line and from its intersection with vertical axis. RESULTS AND DISCUSSION The obtained microstrains are presented in Figures 1b and 2b. It should be pointed out that our experimental widths are not corrected for an instrumental broadening. Therefore, the values of microstrains measured for the pure components give us the instrumental zero points. Let us try to probe deeper. Eqs. (2) and (3) assumes that the microstrain is uniform in all crystallographic directions. This assumption is doubtful for a crystalline material. It is more physical to consider an anisotropic magnitude hkl. In Ref. [9] a uniform stress (deformation pressure) was assumed. In this case in Eqs. (2) and (3) has to be substituted by an anisotropic microstrain hkl=/Ehkl, where Ehkl is the Young's modulus in direction hkl. The Eq. (3) transforms into the Eq. (4): hkl = /( cos hkl) + 4 tan hkl /Ehkl (4) 312 Figure 1. Part a: The lattice parameter for AuxCu1-x and AuxAg1-x metallic alloys presented as a deviation from the Vegard's law a-aVegard. Part b: The microstrain as determined by the XRD for AuxCu1-x and AuxAg1-x metallic alloys. Figure 2. Part a: The lattice parameter for AgClxBr1-x and RbClxBr1-x ionic crystals presented as a deviation from the Vegard's law a-aVegard. Part b: The microstrain as determined by the XRD for AgClxBr1-x and RbClxBr1-x ionic crystals. However, it may be more reasonable to expect the real parameter of deformation to be a density of a deformation energy u. Thus assuming this density being uniform u=(hkl)2Ehkl/2 (Hooke's law), one has to substitute in Eqs. (2) and (3) by the anisotropic microstrain hkl= 2u / Ehkl . As a result one obtains the following equation: hkl = /( cos hkl) + 4 2 / Ehkl tan hkl u (5) Figures 3 and 4 present the comparison of these three evaluation procedures for the Au0.20Cu0.80 and Au0.56Ag0.44 alloys, respectively. Figures 3a and 4a correspond to Eq. (3), Figures 3b and 4b correspond to Eq. (4), Figures 3c and 4c correspond to Eq. (5). Young's modulus Ehkl for cubic crystals [10] were calculated using a linear interpolation of data from Ref. [11]. The scattering of the points from the linear expression is much less for the parts c. All the other concentrations exhibit an analogous behavior. Therefore, the assumption of the uniform deformation energy is found to be better for the metallic samples. As for the mixed salts the broadening effect is an order of magnitude smaller and is too small to see a significant difference between the approaches. 313 Figure 3. The analysis of the angular dependence of the Bragg peaks' widths in assumption of the uniform deformation (part a), uniform stress (part b) and uniform deformation energy u (part c) for Au0.20Cu0.80 alloy. Figure 5 presents the calculated density of the deformation energy for the metallic (part a) and non-metallic (part b) samples. One can see from both figures that the maximal deformation energy density roughly corresponds to the 50-50 concentration range. In substitutionaly disordered crystal each cite may be occupied either by atom of size d 1 or by that of size d2 with probabilities (1-x) and x respectively. The relative size dispersion can be written as [12]: d 2 (1 x)d12 xd22 ((1 x)d1 xd2 ) 2 (d1 d 2 ) 2 x ( 1 x ) 2 ((1 x)d1 xd2 ) 2 d (6) 2 d Indeed, this dispersion has a maximum at 50-50 concentration independent of the disparity of size but its maximal value is greater for the greater disparity. In the case of ionic crystal a distance between anions is a corresponding relevant parameter. Figure 5 demonstrates the correlation between the density of deformation energy and the size dispersion. In spite of the fact that the relative size dispersion (and, simultaneously, the deformation energy) within the ionic crystals is about an order of magnitude less than in the metallic alloys and is within the uncertainty of our measurements, the results do not contradict a positive correlation between u and the size dispersion. 314 Figure 4. The analysis of the angular depandence of the Bragg peaks' widths in assumption of the uniform deformation (part a), uniform stress (part b) and uniform deformation energy u (part c) for Au0.35Ag0.65 alloy. The anisotropy of the microstrains is compatible with our results presented in Figure 6 for lattice parameters ahkl of the Au-Cu alloys evaluated from the positions of the different individual Bragg reflections. The set of ahkl has been calculated for every sample from lattice spacing of corresponding Bragg reflections. The deviations of lattice parameters for different planes from the average depend systematically on concentration. The difference grows rapidly in the midst of th e concentration range while this effect disappears for pure Au and Cu. It means that the effect exceeds considerably the uncertainty of the measurement. Let us stress here the difference in the experimental origins of Figures 5 and 6. While the microstrains are determined by the XRD peaks' widths analysis the lattice parameters ahkl for different crystallographic planes are determined from the peaks' positions. Therefore, the Figures 5 and 6 present the results of two independent experimental techniques. The result presented in Figure 6 originates, probably, in different macroscopic deformations within the grains in different crystallographic directions. This discrepancy is maximal between (111) and (200) planes. It is compatible with the greater stiffness of the direction [111] (space diagonal) in the Au-Cu alloys [10,11]. Thus, the deformation (expansion) in the direction [200] is easier. Such a difference between the lattice parameters for the different crystallographic planes may be considered as a precursor of a transformation of the Au-Cu alloy from the metastable disordered fcc phase to other phases. 315 Figure 5. Part a: The density of the deformation energy u for AuxCu1-x and AuxAg1-x metallic alloys. Dotted (Au-Cu) and dashed (Au-Ag) curves show the relative size dispersion (right scale). Part b: The density of the deformation energy u for AgClxBr1-x and RbClxBr1-x ionic crystals. Dotted (AgClAgBr) and dashed (RbCl-RbBr) curves show the relative size dispersion (right scale). Figure 6. The lattice parameter ahkl evaluated from the positions of the different individual Bragg reflections (presented as a deviation from Vegard's law ahkl-aVegard for AuxCu1-x metallic alloy. The difference in ahkl-aVegard between the (200) and the other (hkl) diffraction peaks is in apparent disagreement with macroscopic cubic symmetry. However, the broadening of the diffraction lines indicates that there is no perfect long range order due to the limited mean "effective" size of the coherent scattering region normal to the reflecting planes and an anisotropic microstrain hkl. Both of these effects broaden the diffraction peaks destroying the long-range cubic symmetry, allowing the microstrains to violate cubic symmetry locally. Another aspect of the results presented here to be noted is that the Au-Cu alloys were rapidly quenched to avoid the thermal equilibrium ordering and phase separation while the Au-Ag alloy and the mixed salts were cooled more slowly, since their disordered phases are thermally stable. Thus, one expects that the quenched alloy would have higher strains than the others, as observed. The microstrains are an inherent feature of the non-thermal equilibrium quenched state and any attempt to anneal them would ca use the alloy to change phase. These microstrains are an inherent feature of the disordered alloy at the lower temperatures and their large magnitude is related to the large relative atomic size disparity in the disordered Au-Cu alloys. The energy associated with the microstrains is the important 316 factor in making the disordered phase of Au-Cu alloys thermally unstable at lower temperatures. CONCLUSIONS 1. 2. 3. A positive correlation exists between both the line positions and the line widths of the XRD pattern and the relative atomic size disparity of the disordered mixed crystals AuCu, Au-Ag, AgCl-AgBr and RbCl-RbBr. Different ways to account the elastic anisotropy of the crystals are compared. It is found that the suggested concept of the uniform density of strain energy u gives the most adequate presentation of our experimental XRD data on the alloys. This strain energy density depends on concentration the same way as the relative atomic size dispersion. The difference between the lattice parameters of Au-Cu alloy evaluated from the different Braggs' reflections (200) and (111) depends on concentration in a systematic way. This fact is interpreted as a precursor of the phase transformation. ACKNOWLEDGEMENTS This work was partially supported by The Aaron Gutwirth Foundation, Allied Investments Ltd.(Israel), by DOE Grant No. DE-FG02-96ER45439 through the Materials Research Laboratory at the University of Illinois at Urbana-Champaign (AIF), and by DOE Grant No. DE-FG03-98ER45681 through the University of Washington, Seattle (AIF and EAS). REFERENCES: 1. A.I.Frenkel, E.A.Stern, A.Voronel, M. Qian, M.Newville, Buckled crystalline structure in mixed ionic salts// Phys. Rev. Lett. 71, 3485 (1993). 2. A.I.Frenkel, E.A.Stern, A.Voronel, M. Qian, M.Newville, Solving the structure of disordered mixed salts// Phys. Rev. B 49, 11662 (1994). 3. A.I.Frenkel, E.A.Stern, A.Voronel, S. Heald, Solid State Commun. 99, 67 (1996). 4. A.I.Frenkel, A.Voronel, A.Katzir, M. Newville, E.A.Stern, Physica B 208&209, 334 (1995). 5. A.I.Frenkel, E.A.Stern, A.Rubshtein, A. Voronel, Yu.Rosenberg, J. Phys. IV, 7, C21005 (1997); A.I.Frenkel, V.Sh.Machavariani, A.Rubshtein, Yu. Rosenberg, A.Voronel, E.A.Stern, Local structure of disordered Au-Cu alloys, in preparation. 6. J.Langford, D.Louer, Rep. Prog. Phys. 59, 131 (1996). 7. G.K.Williamson, W.H.Hall, Acta Metallurgica 1, 22 (1953). 8. H.P.Klug, L.E.Alexander, X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials (NY, Wiley, 1974). 9. K.Reimann and R.Wurschum, J. Appl. Phys. 81, 7186 (1997). 10. A.E.H.Love, A Treatise on the Mathemetical Theory of Elasticity (NY, Dover, 1944). 11. Landolt-Bornstein, Numerical Data and Functional Relationships in Science and Technology, New Series, Volume 11 (Springer-Verlag, Berlin, Heidelberg, NY, 1979). 12. A.Voronel, S.Rabinovich, A.Kisliuk, V. Steinberg, T.Sverbilova, Phys. Rev. Lett. 60, 2402 (1988). 317