Conclusion
advertisement

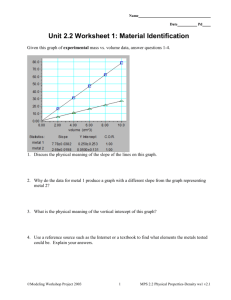
Conclusion It was hypothesized that 2 out of the 3 metals tested would be identified in this lab. However, none of the metals were able to be identified correctly from the data that was collected in the lab; therefore this hypothesis was rejected. It was also hypothesized that the percent error for the metal densities would be no more than 5% from the true metal densities. The percent errors for the lab were 45% for sample X, 63% for sample J and 115% for sample O. Therefore, this hypothesis was also rejected. The reason for such high percent errors might be a result of the measurements taken in the lab being inaccurate. For example, looking at the data collected for sample X it was observed that the densities steadily increased from 4.56g/mL to 6.92g/mL and then to 8.23g/mL. This difference in densities could be the cause of the observed density being so far from the actual density of 4.44g/mL. This increasing experimental error could have been due to not drying the metals well in between the trials, oils and dirt from the experimenter’s hands adding to the weight of the metal, or bubbles on the side of the graduated cylinder that changed the volume of the water, and therefore the sample. Next time these details will be taken into account for each trial to create more accurate measurements. It was also noticed that even though sample J’s measurements were far from the actual density resulting in a 63% error that the densities recorded were actually quite precise. The observed densities were 6.37g/mL, 6.38 g/mL and 6.25g/mL. These numbers are very precise although they are not accurate to the true density of 2.76 g/mL. Since the measurements were actually precise it might be concluded that the group was testing the wrong metal or maybe did not zero out the paper towel on the scale giving much higher numbers than should be expected. Next time, more caution will go into making the measurements accurate as well as precise. References http://www.cde.ca.gov/be/st/ss/scmain.asp (printed 9/23/07)