Binary Molecular Compounds and Acids - for posting
advertisement

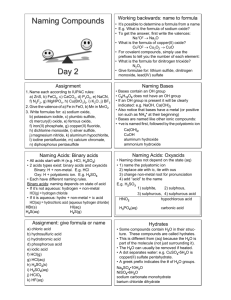
Binary Molecular Compounds Binary Molecular Compounds • Binary molecular compounds are composed of two different nonmetals – examples: CO, SO2, N2H4, P4Cl10 • These compounds are named by using a prefix to indicate the number of atoms of each element present (see table in notebook ). H2O • Dihydrogen Monoxide • The prefix mono- is often omitted especially when the first element would have the prefix monoCO • (example: CO is named carbon monoxide, not monocarbon monoxide). Name the following compounds: NF3 N2O4 P4S10 • NF3 is nitrogen trifluoride • N2O4 is dinitrogen tetroxide (Note: dropped an 'a') • P4S10 is tetraphosphorous decasulfide Write formulas for the following compounds: • dichlorine heptaoxide • carbon hexasulfide • octaphosphorous pentaoxide • dichlorine heptaoxide is Cl2O7 • carbon hexasulfide is CS6 • octaphosphorous pentaoxide is P8O5 Acids Acids • Acids are compounds that give off hydrogen ions, (H+) when dissolved in water. When a compound has hydrogen as its cation the substance is generally an acid – Examples: HCl, H2SO4, H3PO3 • The rules for naming acids are based on the anion portion of the acid formula. Rules for Naming Acids • The names of acids are based on the ending of the anion name. – Examples: HCl, H2SO4, H3PO3 Rules for Naming Acids • The names of acids are based on the ending of the anion name. – Examples: HCl, H2SO4, H3PO3 • Cl‾ = chloride Rules for Naming Acids • The names of acids are based on the ending of the anion name. – Examples: HCl, H2SO4, H3PO3 • Cl‾ = chloride • SO42‾ = sulfate Rules for Naming Acids • The names of acids are based on the ending of the anion name. – Examples: HCl, H2SO4, H3PO3 • Cl‾ = chloride • SO42‾ = sulfate • PO33‾ = phophite Rules for Naming Acids Anion ending Example Acid name Example -ide Cl- (chloride) hydro-(stem)-ic acid hydrochloric acid -ate SO42- (sulfate) (stem)-ic acid sulfuric acid -ite PO33- (phosphite) (stem)-ous acid phosphorous acid Name the acids • HNO2 nitrous acid • HCN hydrocyanic acid • H3PO4 phosphoric acid Write formulas for the following acids • chromic acid H2CrO4 • hydroiodic acid HI • chlorous acid HClO2 Homework • Do the 4 “HOMEWORK” (Not example) problems on the Binary Molecular Compounds and Acids note page. – Do these problems on your own paper. – Homework will be collected tomorrow.