Membrane Transport
advertisement

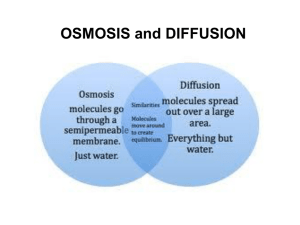
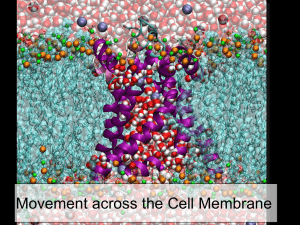
Transport across membranes Learning Outcomes • explain what is meant by passive transport (diffusion and facilitated diffusion including the role of membrane proteins), active transport, endocytosis and exocytosis; Exchange across the plasma membrane • The membrane provides an effective barrier against the movement of substances, however some exchange between the cell and the environment is essential. Transport across membranes • Materials can move across cell membranes: – Passively • Diffusion (simple or facilitated) • Osmosis – Actively • Active transport • Bulk transport Diffusion • Net movement of molecules or ions from a region of high concentration to a region of low concentration • Occurs along a concentration gradient • Result = equilibrium (molecules or ions evenly spread out within a given space or volume) Factors affecting the rate of diffusion • Concentration gradient – Greater the difference in concentration the greater the rate of diffusion • Temperature – At higher temperature kinetic energy particles increases – Diffusion is faster • Surface area – Greater the surface area, more particles can cross – Increases rate of diffusion Factors affecting the rate of diffusion • Nature of molecules or ions – Large molecules diffuse slower – Non-polar molecules diffuse more easily – The respiratory gases (CO2 and O2) are small enough to diffuse quickly through the membrane. – Large, polar molecules (glucose and amino acids) and ions (Na+ and Cl-) cannot diffuse through the phospholipid bilayer Facilitated Diffusion • Protein molecules exist in membranes to facilitate diffusion. • 2 type of protein molecule – Channel protein • transmembrane protein that forms a tunnel through the bilayer. – Carrier proteins • change shape to help molecules move into and out of cells. Facilitated Diffusion Active Transport • Energy consuming transport of molecules or ions across a membrane against a concentration gradient, made possible by transferring energy from respiration. • Energy makes the carrier proteins change shape, transferring ions across the membrane. Examples of active transport • Reabsorption in kidneys • Digestion in gut – Helps absorb glucose from our intestines • Load sugars into phloem • Inorganic ion uptake in root hairs – Magnesium ions are in short supply in the soil but are needed for photosynthesis Bulk transport • This is the method of transporting large quantities of materials into cells (endocytosis) or out of cells (exocytosis) – Endocytosis - Engulfing of material by cell membrane to form a endocytic vacuole. • 2 forms – Phagocytosis the uptake of solid material – Pinocytosis the uptake of liquid – Exocytosis - Process by which materials are removed from cells Examples of bulk transport • Hormones released into bloodstream from endocrine glands • White blood cells engulf invading microorganisms by phagocytosis • In plant cells materials to build the cell wall are carried outside in vesicles. OSMOSIS • Special type of diffusion involving water molecules • Example: – Two solutions are separated by a partially permeable membrane. Solute molecules are too large to pass through pores in the membrane, but water molecules are small enough. What would happen if the membrane were not present? • Net movement of solute molecules from B to A by diffusion • Net movement of water molecules from A to B by diffusion • Equilibrium – concentrations of water molecules and solute molecules in A would equal that in B. What will happen if the membrane is present? What will happen if the membrane is present? • Solute molecules too large to pass through membrane • Water molecules pass easily from A to B • Net movement of water from A to B until equilibrium is reached, i.e. solution A has the same concentration of water molecules as solution B. • The level of liquid A will fall and the level of liquid B will rise • Equilibrium is brought about by the movement of water molecules alone. Definition of osmosis • Water potential Ψ – Tendency of water molecules to diffuse from one place to another. – Measured in kPa – Pure water has a water potential of 0kPa • Osmosis – Is the net movement of water molecules from a region of high water potential to a region of low water potential (down a water potential gradient) across a partially permeable membrane. Water potential Pure water No solute Dilute solution Small amount of solute dissolved Very low water Concentrated potential solution -500kPa Large amount of solute dissolved Lower water potential -50kPa Decreasing water potential Highest water potential 0kPa Some Important Terms • Hypotonic – a region of • higher water potential. • Lower solute concentration • Hypertonic – a region of • lower water potential • Higher solute concentration • Isotonic – a region where there are equal water potentials on either side of a membrane. Determining Water Potential in Potato tubers Salt Soluntion (mol-1) 0.1 0.2 0.3 0.4 0.5 Starting Mass (g) Finishing mass (g) Change in mass (g) %age change in mass Osmosis in Red Blood Cells Osmosis in Plant Cells Important Terms • Turgid – the term used to describe a plant cell where the protoplast exerts a pressure on the cell wall. • Plasmolysed – the term used to describe a plant cell where the protoplast has shrunk away from the cell wall due to loss of water by osmosis. Osmosis in red onion cells