Conservation of Mass
advertisement

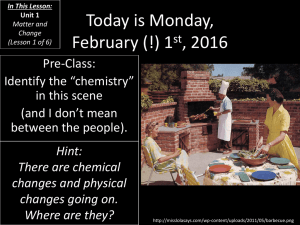
Conservation of Mass Law of Conservation of Mass • The law of conservation of mass states that in any given chemical reaction, the total mass of the reactants equals the total mass of the products. • Chemical equations obey the law of conservation of mass. They show that all the atoms in the reactants are still present in the products. • Coefficients are added before chemical formulas in a chemical equation to ensure that the numbers of atoms on each side of the arrow are equal (balanced) Try These 1. Are the following situations exceptions to the Law of Conservation of mass? Justify your answer in each case. [K] a) b) c) d) The mass of a hamburger decreases as it is barbecued (cooked). A tree’s mass is continually increasing as the tree grows. The mass of a copper penny increases if it is heated in a Bunsen Burner flame. You are often lighter in the morning than you were when you went to bed. 2. You might have noticed that new copper roofs turn green over time. This occurs because copper reacts with substances in the air to form a hard, protective coating. Will the mass of the new copper roof increase or decrease over time? Explain. Does this prediction violate the law of conservation of mass? Explain [I][A] 3. A 20g sample of compound A is mixed with 45g of compound B. A chemical reaction occurs in which a gas is produced. Once the reaction is complete, the final mixture has a mass of 55g. [I] a) b) 4. What is the mass of the gas? What assumption did you make in (a)? Soon after learning about the work of Lavoisier, John Dalton proposed that atoms are never created or destroyed in chemical reactions, only rearranged. Explain how this statement applies to the Law of Conservation of Mass. [A]