Scientific Notation and Significant Figures

Scientific Notation and
Significant Figures
◦ A positive exponent means move the decimal to the right
Ex. 1.34 x 10 4 = 13,400
◦ A negative exponent means move the decimal to the left
Ex. 5.12 x 10 -2 = 0.0512
Now try some!!
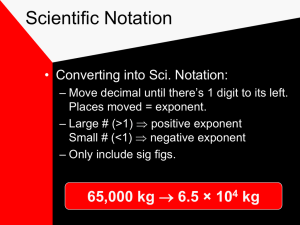
Going from scientific notation to standard number form.
Numbers in scientific notation should begin with a number between 1 and 10 and then should be followed by “x 10” with an exponent.
◦ Large numbers will have a positive exponent
Ex. 67,000 = 6.7 x 10 4
◦ Small numbers will have a negative exponent
Ex. 0.000031 = 3.1 x 10 -5
Now try some!!!
Going from standard number form to scientific notation
Adding/Subtracting Rules
◦ Numbers must have the SAME exponent
◦ Then, just add the numbers as normal and keep the original exponent
Ex. 3.3 x 10 3 + 2.1 x 10 3 = 5.4 x 10 3
Now try some!!!
Math with scientific notation!
What if they are not the same??
o
If exponents are not the same, one must be adjusted o Example: 7.1 x 10 4 – 2.0 x 10 3 o 7.1 x 10 4 can become 71 x 10 3 o 2.0 x 10 3 can become .2 x 10 4
Now try some!!!
Exceptions
Multiplying
◦ When multiplying numbers in scientific notation, the exponents are added
Ex. 3.0 x 10 3 * 2.0 x 10 4 = 6.0 x 10 7
Dividing
◦ When dividing numbers in scientific notation, the exponents are subtracted
Ex. 9.0 x 10 5 / 3.0 x 10 2 = 3.0 x 10 3
Ex. 3.0 x 10 3 / 2.0 x 10 4 = 1.5 x 10 -1
Multiplying and Dividing
Significant Figures
When rounding, we make certain numbers
“insignificant” therefore there are rules with respect to which numbers matter in chemistry
These are called “sig figs”
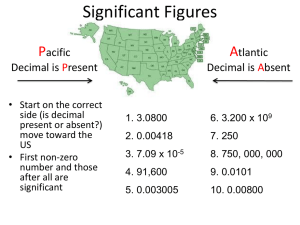
All non-zeros ARE significant
◦ Examples: 1.23 has three sig figs
41.12 has four sig figs
Zeros between non-zeros ARE significant
◦ Examples: 1205 has four sig figs
1.3021 has five sig figs
The Rules
Placeholder zeros are NOT significant
◦ Examples: 34,000 has two sig figs
0.0002 has one sig fig but…. 34,001 has five sig figs… why?
Final zeros after a decimal ARE significant
◦ Examples: 1.200 has four sig figs
34,000.00 has seven sig figs
The Rules
How many sig figs do the following have?
◦ 3.002
◦ 12,000
◦ 12,000.00
◦ 0.009
◦ 12
Now try some!!!
Practice!!
Adding/Subtracting
◦ Answer should have the same number of
DECIMAL PLACES as the original number with the LEAST amount of decimal places
Example: 1.12 + 2.3 = 3.4
2
Math with Sig Figs
Multiplying/Dividing
◦ Answer should have the same number of SIG
FIGS as the original number with the LEAST amount of sig figs.
Examples:
◦ 3.40 x 1.2 = 4.08 4.1
◦ 7 x 24 = 168 200
◦ 14.000 x 2.00 = 28 = 28.0
◦ 45,000 x 112 = 5,040,000 5.0 x 10 6
Math with Sig figs