Solubility & Molarity Notes
advertisement

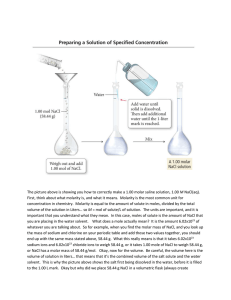
Changes in Solubility I. Pressure A. Changes in pressure do not affect solubility of liquid or solid solutes in liquid solvents. 1. But, increases in pressure do increase the solubility of a gas in liquids. II. Temperature A. Increasing the temperature usually increases the solubility of solids in liquids. B. But, increasing temperature decreases the solubility of gases in liquids. ** This is important for fish in the winter, since colder temperature means more O2 in water which is good since fish move less in cold water. ** Molarity • I. Concentration of Solutions • A. Concentration-DEF- a measure of the amount of solute in a given amount of solvent or solution. • 1. There are two ways in order to express concentration. • a. Molarity • b. Molality • B. Molarity-DEF- the number of moles of solute in one liter of solution. • 1. In order to find the molarity of a solution, you must know the molar mass of the solute. • Ex- a one molar concentration of NaOH would be written- 1M NaOH • 2. Formula • Molarity (M) = amount of solute (mol) • volume of solution (L) • 3. The molarity of any solution can be calculated by dividing the number of moles of solute by the number of liters of solution. • Example #1 • Ex. You have 3.5L of a solution that contains 90.0 g of sodium chloride, NaCl. What is its molarity? • Given: 90.0 g NaCl, 3.5 L • ? – molarity • You NEED moles & Liters…..so you must convert grams to moles. 1 mol NaCl = 58.5 g NaCl (molar mass) * 90.0 g 1 mol NaCl 58.5 g NaCl = 1.54 mol NaCl M = mol/L so… plug in your numbers AND…. M= 1.54 mol NaCl/ 3.5 L of solution (from problem) = 0.44M NaCl (for each 1 liter of solution) • Example #2 • Ex. You have 0.8 L of a 0.5 M HCl solution. How many moles of HCl does this solution contain? • Given: 0.8 L and 0.5 M HCl • ? – moles of HCl • * You need to rearrange the formula for molarity so…. • Moles = volume x molarity • SO…. • moles= 0.8 L x 0.5 M HCl = 0.4 moles of HCl